| Revision as of 13:14, 9 August 2024 editAsonbason (talk | contribs)20 editsm Vandalism CorrectionTags: Reverted Visual edit← Previous edit | Revision as of 08:04, 11 August 2024 edit undo2a00:23c6:19a8:f401:d1e2:e98f:2b02:7f18 (talk) Fixed typo, this is an antiemetic not an antisemeticTags: Reverted Mobile edit Mobile web editNext edit → | ||
| Line 48: | Line 48: | ||
| }} | }} | ||
| '''Trimethobenzamide''' (trade names '''Tebamide''', '''Tigan''') is an ] used to prevent ] and ]. | '''Trimethobenzamide''' (trade names '''Tebamide''', '''Tigan''') is an ] used to prevent ] and ]. | ||
| == Mechanism of action == | == Mechanism of action == | ||
Revision as of 08:04, 11 August 2024
Antiemetic medication Pharmaceutical compound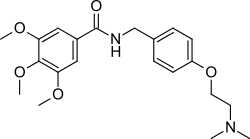 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Tigan, Tebamide |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682693 |
| Routes of administration | Oral, rectal, intramuscular |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 60-100% |
| Elimination half-life | 7 to 9 hours (mean) |
| Excretion | urine (30-50%), faeces |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.848 |
| Chemical and physical data | |
| Formula | C21H28N2O5 |
| Molar mass | 388.464 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Trimethobenzamide (trade names Tebamide, Tigan) is an antiemetic used to prevent nausea and vomiting.
Mechanism of action
Trimethobenzamide is an antagonist of the D2 receptor. It is believed to affect the chemoreceptor trigger zone (CTZ) of the medulla oblongata to suppress nausea and vomiting.
Side effects
Possible side effects include drowsiness, dizziness, headache, muscle cramps, and blurred vision. More serious adverse effects include skin rash, tremors, parkinsonism, and jaundice.
Formulations
Trimethobenzamide is marketed under the brand names Tebamide and Tigan, manufactured by GlaxoSmithKline and King Pharmaceuticals, respectively. It is available as oral capsules and injectable formulations.
Trimethobenzamide was also available as a rectal suppository, but such formulations were banned by the U.S. Food and Drug Administration on April 6, 2007, due to unproven efficacy.
Synthesis

Alkylation of the sodium salt of p-hydroxybenzaldehyde (1) with 2-dimethylaminoethyl chloride affords the ether (2). Reductive amination of the aldehyde in the presence of ammonia gives diamine (3). Acylation of that product with 3,4,5-trimethoxybenzoyl chloride affords trimethobenzamide (4).
See also
References
- Smith HS, Cox LR, Smith BR (2012). "Dopamine receptor antagonists". Ann Palliat Med. 1 (2): 137–42. doi:10.3978/j.issn.2224-5820.2012.07.09. PMID 25841474.
- Waknine, Yael (April 6, 2007). "FDA Bans Suppositories With Trimethobenzamide". Medscape. Retrieved 2007-04-06.