| Revision as of 20:37, 10 April 2024 editRistando (talk | contribs)85 edits Added preparation and safety← Previous edit | Revision as of 13:23, 28 November 2024 edit undo195.245.239.241 (talk) Added missing sodium to sodium adipateTags: Visual edit Mobile edit Mobile web editNext edit → | ||
| Line 52: | Line 52: | ||
| Sodium adipate is prepared by reacting ] with ]:<ref>{{cite web | url = https://healthknight.com/sodium-adipate-side-effects-benefits | title = Sodium Adipate (E356) – Overview, Uses, Side Effects & More | website = healthknight.com }}</ref> | Sodium adipate is prepared by reacting ] with ]:<ref>{{cite web | url = https://healthknight.com/sodium-adipate-side-effects-benefits | title = Sodium Adipate (E356) – Overview, Uses, Side Effects & More | website = healthknight.com }}</ref> | ||
| :<chem>C6H10O4 + 2NaOH -> |
:<chem>C6H10O4 + 2NaOH -> Na2C6H8O4 + 2H2O</chem> | ||
Revision as of 13:23, 28 November 2024
 | |
| Names | |
|---|---|
| Preferred IUPAC name Disodium hexanedioate | |
| Other names
Disodium adipate Adipic acid, sodium salt | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.028.448 |
| E number | E356 (antioxidants, ...) |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C6H8Na2O4 |
| Molar mass | 190.106 g·mol |
| Appearance | Solid white to off-white powder or crystals |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P305+P351+P338 |
| NFPA 704 (fire diamond) |
 |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 4000 mg/kg (intraperitoneal, mouse) |
| Safety data sheet (SDS) | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
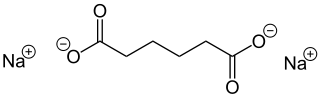
Sodium adipate is a chemical organic compound with formula Na2C6H8O4. It is the sodium salt of adipic acid.
As a food additive, it has the E number E356 as is used as a buffering agent and as an acidity regulator.
Preparation
Sodium adipate is prepared by reacting adipic acid with sodium hydroxide:
Safety
If consumed in excess, it can lead to high levels of sodium and gastrointestinal problems. It can also cause allergic reactions which may lead to swelling, itching, difficulty breathing. Sodium adipate has no proven health benefits.
References
- "E356 Sodium adipate". food-info.net.
- "Sodium Adipate (E356) – Overview, Uses, Side Effects & More". healthknight.com.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |
