| Revision as of 14:52, 29 April 2009 edit71.174.189.14 (talk)No edit summary← Previous edit | Revision as of 01:39, 12 May 2009 edit undo71.123.114.190 (talk) cleaned up articleNext edit → | ||
| Line 2: | Line 2: | ||
| {{mergeto|Atomic orbital|Talk:Electron cloud#Merger proposal|date=July 2008}} | {{mergeto|Atomic orbital|Talk:Electron cloud#Merger proposal|date=July 2008}} | ||
| {{about|the structure of an atom|the particle accelerator phenomenon|Electron-Cloud Effect}} | {{about|the structure of an atom|the particle accelerator phenomenon|Electron-Cloud Effect}} | ||
| '''Electron cloud''' is a term used, if not originally coined, by the ] laureate and acclaimed educator ] in ] (] Vol 1 lect 6 pg 11) for discussing "exactly what is an ]?". This intuitive model provides a simplified way of visualizing an electron as a solution of the ], an advancement using the ] to surprising observations that could only be explained by introducing randomness. It is also often referred to as an orbital, because the two terms similarly conceptualize the space where an electron is likely to be found but cannot be actually pinpointed. In the electron cloud analogy, the ] of an electron, or ], is described as a small cloud moving around the ] or molecular ], with the opacity of the cloud proportional to the probability density. | |||
| ] of hydrogen, with a plus and a minus sign superimposed. The plus sign represents the location of the nucleus; the minus sign represents a possible location of the electron.]] | |||
| ⚫ | The model evolved from the earlier ], which likened an electron |
||
| '''Electron cloud''' is a term used, if not originally coined, by the ] laureate and acclaimed educator ] in ] (] Vol 1 lect 6 pg 11) for discussing "exactly what is an ]?". In the electron cloud analogy, an electron is described as a cloud surrounding the ] of an atom (or the nuclei of the atoms in a molecule). The thicker the cloud is in a region, the more likely that the electron, when its position is ], will be found there. Electron clouds allows one to visualize the random nature of the position of quantum particles, as opposed to classical particles which can be located at one point. | |||
| ⚫ | Experimental evidence suggests that the probability density is not just a theoretical model for the uncertainty in the location of the electron, but rather that it reflects the actual state of the electron. This carries an enormous philosophical implication, indicating that point-like particles do not actually exist, and that the universe's evolution may be fundamentally uncertain. The fundamental source of quantum uncertainty is an ] in physics. |
||
| To go into more detail, this intuitive model provides a way of visualizing an electron as a solution of the ]. Solutions of Schrödinger's equation are called ], and when one takes the square of the absolute value of the wavefunction, one obtains the ] of the position of an electron. A three-dimensional plot of the probability density, where the opacity of the cloud is proportional to the probability density, gives us the electron cloud image. | |||
| In the electron cloud model, rather than following fixed orbits, electrons bound to an atom are observed more frequently in certain areas around the nucleus called ]s. The electron cloud can transition between ] states, and each state has a characteristic shape and energy, all predicted by the Schrödinger equation, which has infinitely many solutions. Experimental results motivated this conceptual refinement of the Bohr model. The electron cloud model is a newer, better version of the Bohr model. It shows all the electrons moving around, not just in an orbit.The famous double slit experiment demonstrates the random behavior of electrons, | |||
| ⚫ | The electron cloud model evolved from the earlier ], which likened an electron surrounding an atomic nucleus to a planet ]ing the sun. From the Bohr model we get the term ]. The electron cloud model better describes many observed phenomena, including the ], the ] and ], and atomic interactions with light. This model demonstrates the ] of an electron, in that electron behavior is described as a delocalized wavelike object. | ||
| Physicist Adam Wagner speculates that, if visible, an electron cloud would look much like the static on a television screen. | |||
| ⚫ | Experimental evidence suggests that the probability density is not just a theoretical model for the uncertainty in the location of the electron, but rather that it reflects the actual state of the electron.{{fact}} This carries an enormous philosophical implication, indicating that point-like particles do not actually exist, and that the universe's evolution may be fundamentally uncertain. The fundamental source of quantum uncertainty is an ] in physics. ] is one relatively recent attempt to shed light on this question, and has met with only partial success so far. | ||
| == See Also == | |||
| * ] | |||
| == References == | == References == | ||
Revision as of 01:39, 12 May 2009
| This article may require cleanup to meet Misplaced Pages's quality standards. No cleanup reason has been specified. Please help improve this article if you can. (October 2008) (Learn how and when to remove this message) |
| It has been suggested that this article be merged into Atomic orbital and Talk:Electron cloud#Merger proposal. (Discuss) Proposed since July 2008. |
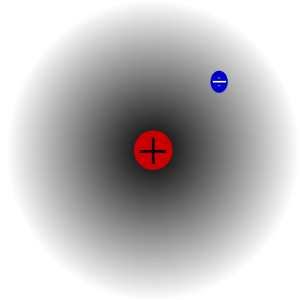
Electron cloud is a term used, if not originally coined, by the Nobel Prize laureate and acclaimed educator Richard Feynman in The Feynman Lectures on Physics (Feynman2006 Vol 1 lect 6 pg 11) for discussing "exactly what is an electron?". In the electron cloud analogy, an electron is described as a cloud surrounding the nucleus of an atom (or the nuclei of the atoms in a molecule). The thicker the cloud is in a region, the more likely that the electron, when its position is measured, will be found there. Electron clouds allows one to visualize the random nature of the position of quantum particles, as opposed to classical particles which can be located at one point.
To go into more detail, this intuitive model provides a way of visualizing an electron as a solution of the Schrödinger equation. Solutions of Schrödinger's equation are called wavefunctions, and when one takes the square of the absolute value of the wavefunction, one obtains the probability density of the position of an electron. A three-dimensional plot of the probability density, where the opacity of the cloud is proportional to the probability density, gives us the electron cloud image.
The electron cloud model evolved from the earlier Bohr model, which likened an electron surrounding an atomic nucleus to a planet orbiting the sun. From the Bohr model we get the term orbitals. The electron cloud model better describes many observed phenomena, including the double slit experiment, the periodic table and chemical bonding, and atomic interactions with light. This model demonstrates the wave nature of an electron, in that electron behavior is described as a delocalized wavelike object.
Experimental evidence suggests that the probability density is not just a theoretical model for the uncertainty in the location of the electron, but rather that it reflects the actual state of the electron. This carries an enormous philosophical implication, indicating that point-like particles do not actually exist, and that the universe's evolution may be fundamentally uncertain. The fundamental source of quantum uncertainty is an unsolved problem in physics. Stochastic Electrodynamics is one relatively recent attempt to shed light on this question, and has met with only partial success so far.
See Also
References
- Feynman, Richard; Leighton; Sands. (2006). The Feynman Lectures on Physics -The Definitive Edition- . Pearson Addison Wesley. ISBN 0-8053-9046-4
- Allen, Stanley H. Photoelectricity - The Liberation of Electrons By Light