| Revision as of 17:54, 8 October 2009 editBeetstra (talk | contribs)Edit filter managers, Administrators172,044 edits clear fake entry for CASNo← Previous edit | Revision as of 23:22, 16 January 2010 edit undoProjecttobe (talk | contribs)2 editsm http://209.85.129.132/search?q=cache:Y3GE800ZWzsJ:www.inchem.org/documents/ehc/ehc/ehc092.htm+RESMETHRIN+%2B+1967&cd=4&hl=ar&ct=clnk&gl=egNext edit → | ||
| Line 23: | Line 23: | ||
| ==References== | ==References== | ||
| {{reflist}} | {{reflist}} | ||
| Resmethrin was first produced in 1967 by Elliott et al. (1967). It | |||
| is prepared by the esterification of [1RS, 3RS or 1RS, cis, | |||
| trans]-2,2-dimethyl-3-(2,2-dimethylvinyl)cyclopropanecarboxylic acid | |||
| or chrysanthemic acid with 5-benzyl-3-furyl-methyl alcohol. | |||
| ==External links== | ==External links== | ||
Revision as of 23:22, 16 January 2010
 | |
| Names | |
|---|---|
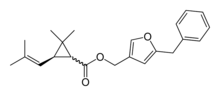
| IUPAC name 5-benzyl-3-[({[(3R)-2,2-dimethyl-3-(2-methylprop-1-en-1-yl) cyclopropyl]carbonyl}oxy)methyl]furan | |
| Identifiers | |
| 3D model (JSmol) | |
| ECHA InfoCard | 100.030.842 |
| CompTox Dashboard (EPA) | |
SMILES
| |
| Properties | |
| Chemical formula | C22H26O3 |
| Molar mass | 338.44 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Resmethrin is a pyrethroid insecticide with many uses, including control of the adult mosquito population. A commercial trade names for products that contain resmethrin are Chrysron, Crossfire, Pynosect, Raid Flying Insect Killer, Scourge, Sun-Bugger #4, SPB-1382, Synthrin, Syntox, Vectrin and Whitmire PT-110.
References
- Pesticide Information Profiles, Extension Toxicology Network (EXTOXNET). Resmethrin
Resmethrin was first produced in 1967 by Elliott et al. (1967). It
is prepared by the esterification of [1RS, 3RS or 1RS, cis, trans]-2,2-dimethyl-3-(2,2-dimethylvinyl)cyclopropanecarboxylic acid or chrysanthemic acid with 5-benzyl-3-furyl-methyl alcohol.
External links
- Resmethrin Technical Fact Sheet - National Pesticide Information Center
- Pyrethrins and Pyrethroids Fact Sheet - National Pesticide Information Center
- Resmethrin Pesticide Information Profile - Extension Toxicology Network
- MSDS for Scourge' Formula II
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |