| Revision as of 14:16, 18 June 2010 edit198.119.145.175 (talk) I deleted "and emits". Atmospheric carbon dioxide absorbs infrared radiation at the mentioned wavelengths. It emits a black body spectrum.← Previous edit | Revision as of 14:18, 18 June 2010 edit undoWilliam M. Connolley (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers66,041 edits Undid revision 368798567 by 198.119.145.175 (talk) no, it doesn't emit at blackbody. say "kirchoff"Next edit → | ||
| Line 7: | Line 7: | ||
| There is an annual fluctuation of about 3–9 ppmv which roughly follows the Northern Hemisphere's growing season. The ] dominates the annual cycle of CO<sub>2</sub> concentration because it has much greater land area and plant biomass than the Southern Hemisphere. Concentrations peak in May as the Northern Hemisphere spring greenup begins and reach a minimum in October when the quantity of ] undergoing photosynthesis is greatest.<ref></ref> | There is an annual fluctuation of about 3–9 ppmv which roughly follows the Northern Hemisphere's growing season. The ] dominates the annual cycle of CO<sub>2</sub> concentration because it has much greater land area and plant biomass than the Southern Hemisphere. Concentrations peak in May as the Northern Hemisphere spring greenup begins and reach a minimum in October when the quantity of ] undergoing photosynthesis is greatest.<ref></ref> | ||
| Despite its relatively small concentration overall in the atmosphere, CO<sub>2</sub> is an important component of Earth's atmosphere because it absorbs ] radiation at ]s of 4.26 ] (asymmetric stretching ]) and 14.99 µm (bending vibrational mode), thereby playing a |
Despite its relatively small concentration overall in the atmosphere, CO<sub>2</sub> is an important component of Earth's atmosphere because it absorbs and emits ] radiation at ]s of 4.26 ] (asymmetric stretching ]) and 14.99 µm (bending vibrational mode), thereby playing a role in the ].<ref>Petty, G.W.: ''A First Course in Atmospheric Radiation'', pages 229–251, Sundog Publishing, 2004</ref> | ||
| ==Sources of carbon dioxide== | ==Sources of carbon dioxide== | ||
Revision as of 14:18, 18 June 2010
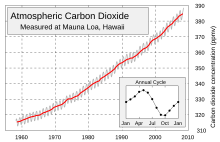
The concentration of carbon dioxide (CO2) in Earth's atmosphere is approximately 390 ppm by volume as of 2010, and rising by about 1.9 ppm/yr. Carbon dioxide is essential to photosynthesis in plants and other photoautotrophs, and is also a prominent greenhouse gas. The present level is higher than at any time during the last 800 Ka, and likely higher than in the past 20 Ma.
Current concentration
In 2009, the CO2 global average concentration in Earth's atmosphere was about 0.0387% by volume, or 387 parts per million by volume (ppmv). This is 103 ppmv (36%) above the 1832 antarctic ice core levels of 284 ppmv. There is an annual fluctuation of about 3–9 ppmv which roughly follows the Northern Hemisphere's growing season. The Northern Hemisphere dominates the annual cycle of CO2 concentration because it has much greater land area and plant biomass than the Southern Hemisphere. Concentrations peak in May as the Northern Hemisphere spring greenup begins and reach a minimum in October when the quantity of biomass undergoing photosynthesis is greatest.
Despite its relatively small concentration overall in the atmosphere, CO2 is an important component of Earth's atmosphere because it absorbs and emits infrared radiation at wavelengths of 4.26 µm (asymmetric stretching vibrational mode) and 14.99 µm (bending vibrational mode), thereby playing a role in the greenhouse effect.
Sources of carbon dioxide

Natural sources of atmospheric carbon dioxide include volcanic outgassing, the combustion of organic matter, and the respiration processes of living aerobic organisms; man-made sources of carbon dioxide include the burning of fossil fuels for heating, power generation and transport, as well as some industrial processes such as cement making. It is also produced by various microorganisms from fermentation and cellular respiration. Plants convert carbon dioxide to carbohydrates during a process called photosynthesis. They produce the energy needed for this reaction through the photolysis of water. The resulting gas, oxygen, is released into the atmosphere by plants, which is subsequently used for respiration by heterotrophic organisms, forming a cycle.

Over 95% of total CO2 emissions are natural. For example, the natural decay of organic material in forests and grasslands, such as dead trees, results in the release of about 220 gigatonnes of carbon dioxide every year. In 1997, Indonesian peat fires were estimated to have released between 13% and 40% of the average carbon emissions caused by the burning of fossil fuels around the world in a single year. Although the initial carbon dioxide in the atmosphere of the young Earth was produced by volcanic activity, modern volcanic activity releases only 130 to 230 megatonnes of carbon dioxide each year, which is less than 1% of the amount released by human activities.
These natural sources are nearly balanced by natural sinks, physical and biological processes which remove carbon dioxide from the atmosphere. For example, some carbon dioxide dissolves in sea water, and some is removed by plants via photosynthesis.
There is a large natural flux of CO2 into and out of the biosphere and oceans. In the pre-industrial era these fluxes were largely in balance. Currently about 57% of human-emitted CO2 is removed by the biosphere and oceans. The ratio of the increase in atmospheric CO2 to emitted CO2 is known as the airborne fraction (Keeling et al., 1995); this varies for short-term averages but is typically about 45% over longer (5 year) periods.
Burning fossil fuels such as coal and petroleum is the leading cause of increased anthropogenic CO2; deforestation is the second major cause. In 2008, 8.67 gigatonnes of carbon (31.8 gigatonnes of CO2) were released from fossil fuels worldwide, compared to 6.14 gigatonnes in 1990. In addition, land use change contributed 1.20 gigatonnes in 2008, compared to 1.64 gigatonnes in 1990.
This addition, about 3% of annual natural emissions as of 1997, is sufficient to exceed the balancing effect of sinks. As a result, carbon dioxide has gradually accumulated in the atmosphere, and as of 2008, its concentration is 38% above pre-industrial levels.
Various techniques have been proposed for removing excess carbon dioxide from the atmosphere in carbon dioxide sinks.
Historical variation
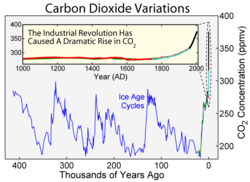
The most direct method for measuring atmospheric carbon dioxide concentrations for periods before direct sampling is to measure bubbles of air (fluid or gas inclusions) trapped in the Antarctic or Greenland ice caps. The most widely accepted of such studies come from a variety of Antarctic cores and indicate that atmospheric CO2 levels were about 260 – 280 ppmv immediately before industrial emissions began and did not vary much from this level during the preceding 10,000 years (10 ka).
One study disputed the claim of stable CO2 levels during the present interglacial of the last 10 ka. Based on an analysis of fossil leaves, Wagner et al. argued that CO2 levels during the period 7 – 10 ka were significantly higher (~300 ppm) and contained substantial variations that may be correlated to climate variations. Others have disputed such claims, suggesting they are more likely to reflect calibration problems than actual changes in CO2. Relevant to this dispute is the observation that Greenland ice cores often report higher and more variable CO2 values than similar measurements in Antarctica. However, the groups responsible for such measurements (e.g., Smith et al.) believe the variations in Greenland cores result from in situ decomposition of calcium carbonate dust found in the ice. When dust levels in Greenland cores are low, as they nearly always are in Antarctic cores, the researchers report good agreement between Antarctic and Greenland CO2 measurements.
The longest ice core record comes from East Antarctica, where ice has been sampled to an age of 800 ka. During this time, the atmospheric carbon dioxide concentration has varied by volume between 180 – 210 ppm during ice ages, increasing to 280 – 300 ppm during warmer interglacials.

On long timescales, atmospheric CO2 content is determined by the balance among geochemical processes including organic carbon burial in sediments, silicate rock weathering, and volcanism. The net effect of slight imbalances in the carbon cycle over tens to hundreds of millions of years has been to reduce atmospheric CO2. The rates of these processes are extremely slow; hence they are of limited relevance to the atmospheric CO2 response to emissions over the next hundred years.
Various proxy measurements have been used to attempt to determine atmospheric carbon dioxide levels millions of years in the past. These include boron and carbon isotope ratios in certain types of marine sediments, and the number of stomata observed on fossil plant leaves. While these measurements give much less precise estimates of carbon dioxide concentration than ice cores, there is evidence for very high CO2 volume concentrations between 200 and 150 Ma of over 3,000 ppm and between 600 and 400 Ma of over 6,000 ppm. In more recent times, atmospheric CO2 concentration continued to fall after about 60 Ma. About 34 Ma, the time of the Eocene-Oligocene extinction event and when the Antarctic ice sheet started to take its current form, CO2 is found to have been about 760 ppm, and there is geochemical evidence that volume concentrations were less than 300 ppm by about 20 Ma. Low CO2 concentrations may have been the stimulus that favored the evolution of C4 plants, which increased greatly in abundance between 7 and 5 Ma.
Relationship with oceanic concentration
See also: Solubility pump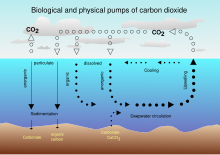
The Earth's oceans contain a huge amount of carbon dioxide in the form of bicarbonate and carbonate ions — much more than the amount in the atmosphere. The bicarbonate is produced in reactions between rock, water, and carbon dioxide. One example is the dissolution of calcium carbonate:
- CaCO3 + CO2 + H2O ⇌ Ca + 2 HCO3
Reactions like this tend to buffer changes in atmospheric CO2. Since the right-hand side of the reaction produces an acidic compound, adding CO2 on the left-hand side decreases the pH of sea water, a process which has been termed ocean acidification. Reactions between carbon dioxide and non-carbonate rocks also add bicarbonate to the seas. This can later undergo the reverse of the above reaction to form carbonate rocks, releasing half of the bicarbonate as CO2. Over hundreds of millions of years this has produced huge quantities of carbonate rocks.
Ultimately, most of the CO2 emitted by human activities will dissolve in the ocean, however the rate at which the ocean will take it up in the future is less certain.
See also
- Avoiding Dangerous Climate Change - A Scientific Symposium on Stabilisation of Greenhouse Gases
- Carbon cycle
- Carbon dioxide equivalent
- Global warming
- Greenhouse effect
- List of countries by carbon dioxide emissions per capita
- List of countries by carbon dioxide emissions
- List of countries by ratio of GDP to carbon dioxide emissions
- Ocean acidification
References
- ^ Tans, Pieter. "Trends in Carbon Dioxide". NOAA/ESRL. Retrieved 2009-12-11.
- ^ http://www.globalcarbonproject.org/carbonbudget/08/hl-full.htm Carbon Budget 2008 Highlights
- ^ "Deep ice tells long climate story". BBC News. 2006-09-04. Retrieved 2010-04-28.
- ^ Climate Change 2001: The Scientific Basis
- "Current atmospheric CO2 concentration at http://co2unting.com".
{{cite web}}: External link in|title= - "Historical CO2 record derived from a spline fit (20 year cutoff) of the Law Dome DE08 and DE08-2 ice cores". Retrieved 2007-06-12.
- Carbon Dioxide Information Analysis Center (CDIAC) - Frequently Asked Questions
- Petty, G.W.: A First Course in Atmospheric Radiation, pages 229–251, Sundog Publishing, 2004
- Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1038/nature01131, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1038/nature01131instead. - Indonesian Wildfires Accelerated Global Warming
- Massive peat burn is speeding climate change - 06 November 2004 - New Scientist
- Gerlach, T.M., 1992, Present-day CO2 emissions from volcanoes: Eos, Transactions, American Geophysical Union, Vol. 72, No. 23, June 4, 1991, pp. 249, and 254 – 255
- U.S. Geological Survey, "Volcanic Gases and Their Effects" http://volcanoes.usgs.gov/Hazards/What/VolGas/volgas.html
- http://www.pnas.org/content/104/47/18866.abstract
- ^ http://lgmacweb.env.uea.ac.uk/lequere/co2/carbon_budget.htm Global carbon budget 2008
- US Global Change Research Information Office, "Common Questions about Climate Change" http://www.gcrio.org/ipcc/qa/05.html
- Wagner, Friederike (2002). "Rapid atmospheric O2 changes associated with the 8,200-years-B.P. cooling event". PNAS. 99 (19): 12011–12014. doi:10.1073/pnas.182420699. PMID 12202744.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Indermühle, Andreas (1999). "Early Holocene Atmospheric CO2 Concentrations". Science. 286 (5446): 1815. doi:10.1126/science.286.5446.1815a. Retrieved May 26, 2005.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Smith, H.J. (1997). "The CO2 concentration of air trapped in GISP2 ice from the Last Glacial Maximum-Holocene transition". Geophysical Research Letters. 24 (1): 1–4. doi:10.1029/96GL03700.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Chemical & Engineering News: Latest News - Ice Core Record Extended
- ncdc.noaa.gov
- New CO2 data helps unlock the secrets of Antarctic formation September 13th, 2009
- Archer, D. (2005). Fate of fossil fuel CO2 in geologic time. J. Geophys. Res., 110, doi:10.1029/2004JC002625.