| Revision as of 11:52, 20 March 2010 editCitation bot (talk | contribs)Bots5,431,806 editsm Citation maintenance. Added: doi, last2, first2, last3, first3, last4, first4, last5, first5. Rjwilmsi← Previous edit | Revision as of 06:35, 28 June 2010 edit undoCitation bot 1 (talk | contribs)Bots130,044 editsm Citations: added: publisher. You can use this bot yourself! Report bugs here.Next edit → | ||
| Line 25: | Line 25: | ||
| ==Occurrence in Lichen== | ==Occurrence in Lichen== | ||
| Together with ] and ], vulpinic acid is secondary metabolite of the fungi. It is speculated that the substances are used as repellent for some herbivores.<ref>{{cite journal | first = James D. | last = Lawrey | journal = The Bryologist | volume = 92 | issue = 3 | year = 1989 | pages = 326–328 |url = http://www.jstor.org/stable/3243401 | title = Lichen Secondary Compounds: Evidence for a Correspondence between Antiherbivore and Antimicrobial Function | doi = 10.2307/3243401}}</ref> The substance showed also some activity against gram-positive bacteria. <ref>{{cite journal |doi = 10.1017/S0024282992000574 |title = The Influence of pH and Lichen Metabolites (Vulpinic Acid and (+) Usnic Acid) on the Growth of the Lichen Photobiont Trebouxia Irregularis |year = 1998 |author = Bačkor, M. |journal = The Lichenologist |volume = 30 |pages = 577 |last2 = Hudá |first2 = J. |last3 = Repčák |first3 = M. |last4 = Ziegler§ |first4 = W. |last5 = Bačkorová |first5 = M.}}</ref> | Together with ] and ], vulpinic acid is secondary metabolite of the fungi. It is speculated that the substances are used as repellent for some herbivores.<ref>{{cite journal | first = James D. | last = Lawrey | journal = The Bryologist | volume = 92 | issue = 3 | year = 1989 | pages = 326–328 |url = http://www.jstor.org/stable/3243401 | title = Lichen Secondary Compounds: Evidence for a Correspondence between Antiherbivore and Antimicrobial Function | doi = 10.2307/3243401 | publisher = The Bryologist, Vol. 92, No. 3}}</ref> The substance showed also some activity against gram-positive bacteria. <ref>{{cite journal |doi = 10.1017/S0024282992000574 |title = The Influence of pH and Lichen Metabolites (Vulpinic Acid and (+) Usnic Acid) on the Growth of the Lichen Photobiont Trebouxia Irregularis |year = 1998 |author = Bačkor, M. |journal = The Lichenologist |volume = 30 |pages = 577 |last2 = Hudá |first2 = J. |last3 = Repčák |first3 = M. |last4 = Ziegler§ |first4 = W. |last5 = Bačkorová |first5 = M.}}</ref> | ||
| ==References== | ==References== | ||
Revision as of 06:35, 28 June 2010
| |||
| Names | |||
|---|---|---|---|
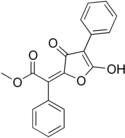
| IUPAC name Methyl (2E)-2-(5-hydroxy-3-oxo-4-phenylfuran-2-ylidene)-2-phenylacetate | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ECHA InfoCard | 100.007.560 | ||
| PubChem CID | |||
SMILES
| |||
| Properties | |||
| Chemical formula | C19H14O5 | ||
| Molar mass | 322.316 g·mol | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |||
Vulpinic acid is a naturally occurring pulvinic acid derivative found in several lichen species, as well as some non-lichenized fungi. It was first isolated in 1925. It is bright yellow, and relatively toxic.
Occurrence in Lichen
Together with usnic acid and pinastric acid, vulpinic acid is secondary metabolite of the fungi. It is speculated that the substances are used as repellent for some herbivores. The substance showed also some activity against gram-positive bacteria.
References
- Mazza, Franc Paolo (1925). "Constitution and physical properties of vulpinic acid". Rend. Accad. Sci. Napoli. 31: 182–90.
- Lawrey, James D. (1989). "Lichen Secondary Compounds: Evidence for a Correspondence between Antiherbivore and Antimicrobial Function". The Bryologist. 92 (3). The Bryologist, Vol. 92, No. 3: 326–328. doi:10.2307/3243401.
- Bačkor, M.; Hudá, J.; Repčák, M.; Ziegler§, W.; Bačkorová, M. (1998). "The Influence of pH and Lichen Metabolites (Vulpinic Acid and (+) Usnic Acid) on the Growth of the Lichen Photobiont Trebouxia Irregularis". The Lichenologist. 30: 577. doi:10.1017/S0024282992000574.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |