| Revision as of 10:15, 3 August 2010 editMattyjenjen (talk | contribs)327 editsNo edit summary← Previous edit | Revision as of 18:29, 11 September 2010 edit undoCitation bot 1 (talk | contribs)Bots130,044 editsm Citations: Formatted dashes. You can use this bot yourself. Report bugs here.Next edit → | ||
| Line 27: | Line 27: | ||
| }} | }} | ||
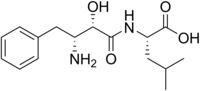
| '''Ubenimex''' (]), also known as '''bestatin''', is a competitive ]. It is an ] of aminopeptidase B<ref>{{cite journal | year = 1976 | issue = 29 | pages = |
'''Ubenimex''' (]), also known as '''bestatin''', is a competitive ]. It is an ] of aminopeptidase B<ref>{{cite journal | year = 1976 | issue = 29 | pages = 97–99 | title = Bestatin, an inhibitor of aminopeptidase B, produced by actinomycetes.| author = Umezawa,H., Aoyagi,T., Suda,H., Hamada,M. & Takeuchi,T.}}</ref>, ] <ref>{{cite journal | year = 1994 | issue = 48 | pages = 131–137 | title = Modulation of pulmonary leukotriene formation and perfusion pressure by bestatin, an inhibitor of leukotriene A4 hydrolase.| author = Muskardin,D.T., Voelkel,N.F. & Fitzpatrick,F.A.}}</ref>, ].<ref>{{cite journal | year = 1999 | issue = 5 | pages = 729–734 | title = Induction of apoptosis by bestatin (ubenimex) in human leukemic cell lines | author = K Sekine, H Fujii and F Abe | volume = 13}}</ref> It is being studied for use in the treatment of ].<ref>{{Cite journal | pmid = 12938265 | year = 2003 | last1 = Hirayama | first1 = Y | last2 = Sakamaki | first2 = S | last3 = Takayanagi | first3 = N | last4 = Tsuji | first4 = Y | last5 = Sagawa | first5 = T | last6 = Chiba | first6 = H | last7 = Matsunaga | first7 = T | last8 = Niitsu | first8 = Y | title = Chemotherapy with ubenimex corresponding to patient age and organ disorder for 18 cases of acute myelogeneous leukemia in elderly patients--effects, complications and long-term survival | volume = 30 | issue = 8 | pages = 1113–8 | journal = Gan to kagaku ryoho. Cancer & chemotherapy}}</ref> | ||
| .]] | .]] | ||
Revision as of 18:29, 11 September 2010
 | |
| Names | |
|---|---|
| IUPAC name (2S)-2-amino]-4-methylpentanoic acid | |
| Other names Bestatin; N--L-leucine | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ECHA InfoCard | 100.055.917 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
SMILES
| |
| Properties | |
| Chemical formula | C16H24N2O4 |
| Molar mass | 308.378 g·mol |
| Melting point | 245 °C (dec.) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Ubenimex (INN), also known as bestatin, is a competitive protease inhibitor. It is an inhibitor of aminopeptidase B, leukotriene A4 hydrolase , aminopeptidase N. It is being studied for use in the treatment of acute myelocytic leukemia.
References
- N-((2S,3R)-3-Amino-2-hydroxy-4-phenylbutyryl)-L-leucine at Sigma-Aldrich
- Umezawa,H., Aoyagi,T., Suda,H., Hamada,M. & Takeuchi,T. (1976). "Bestatin, an inhibitor of aminopeptidase B, produced by actinomycetes" (29): 97–99.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - Muskardin,D.T., Voelkel,N.F. & Fitzpatrick,F.A. (1994). "Modulation of pulmonary leukotriene formation and perfusion pressure by bestatin, an inhibitor of leukotriene A4 hydrolase" (48): 131–137.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - K Sekine, H Fujii and F Abe (1999). "Induction of apoptosis by bestatin (ubenimex) in human leukemic cell lines". 13 (5): 729–734.
{{cite journal}}: Cite journal requires|journal=(help) - Hirayama, Y; Sakamaki, S; Takayanagi, N; Tsuji, Y; Sagawa, T; Chiba, H; Matsunaga, T; Niitsu, Y (2003). "Chemotherapy with ubenimex corresponding to patient age and organ disorder for 18 cases of acute myelogeneous leukemia in elderly patients--effects, complications and long-term survival". Gan to kagaku ryoho. Cancer & chemotherapy. 30 (8): 1113–8. PMID 12938265.
This pharmacology-related article is a stub. You can help Misplaced Pages by expanding it. |