| Revision as of 15:54, 24 February 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{drugbox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref') per Chem/Drugbox validation (report [[Misplaced Pages talk:WikiProj← Previous edit |
Revision as of 17:55, 25 February 2011 edit undoAnypodetos (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers39,350 edits Pregnancy; additional trade name; copyeditNext edit → |
| Line 16: |
Line 16: |
|
| KEGG = D01993 |
|
| KEGG = D01993 |
|
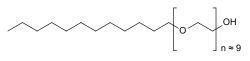
| C= 30 | H= 62 | O= 10 |
|
| C= 30 | H= 62 | O= 10 |
|
| molecular_weight = 583 g/mol (average) |
|
| molecular_weight = ~600 g/mol (average) |
|
| smiles = |
|
| smiles = |
|
| synonyms = |
|
| synonyms = |
| Line 22: |
Line 22: |
|
*Laureth 9 |
|
*Laureth 9 |
|
*Macrogol lauryl ether |
|
*Macrogol lauryl ether |
|
|
*Lauromacrogol |
|
*Lauromacrogols |
|
|
*PEG-9 lauryl alcohol |
|
*PEG-9 lauryl alcohol |
|
*POE-9 lauryl alcohol |
|
*POE-9 lauryl alcohol |
| Line 46: |
Line 46: |
|
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
|
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
|
| pregnancy_US = <!-- A / B / C / D / X --> |
|
| pregnancy_US = <!-- A / B / C / D / X --> |
|
| pregnancy_category= |
|
| pregnancy_category= Topical: allowed<br />Injection: contraindication in months 1–3 and after week 36 |
|
| legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled--> |
|
| legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled--> |
|
| legal_CA = <!-- Schedule I, II, III, IV, V, VI, VII, VIII --> |
|
| legal_CA = <!-- Schedule I, II, III, IV, V, VI, VII, VIII --> |
| Line 55: |
Line 55: |
|
| routes_of_administration = |
|
| routes_of_administration = |
|
}} |
|
}} |
|
'''Polidocanol''' is a ] and ] component of creams and bath additives. It relieves itching caused fr example by ] conditions such as ].<ref>{{cite web | url = http://www.netdoctor.co.uk/medicines/100004774.html | title = E45 itch relief cream |
|
'''Polidocanol''' is a ] and ] component of ]s and bath additives. It relieves itching caused for example by ] conditions such as ].<ref>{{cite web | url = http://www.netdoctor.co.uk/medicines/100004774.html | title = E45 itch relief cream |
|
| publisher = netdoctor.co.uk | accessdate = 2007-07-12}}</ref> |
|
| publisher = netdoctor.co.uk | accessdate = 2007-07-12}}</ref> |
|
|
|
|
|
The substance is also used as a ], an ] injected to treat ], under the trade name '''Asclera'''.<ref>, Laurence Z Rosenberg, MD, eMedicine.com</ref> Polidocanol causes ] inside varicose veins, occluding the ] of the vessel, and reducing the appearance of the varicosity. |
|
The substance is also used as a ], an ] injected to treat ], under the trade names '''Asclera''' and '''Aethoxysklerol'''.<ref>, Laurence Z Rosenberg, MD, eMedicine.com</ref> Polidocanol causes ] inside varicose veins, occluding the ] of the vessel, and reducing the appearance of the varicosity. |
|
|
|
|
|
The FDA has approved polidocanol injections for the treatment of small varicose (less than 1 mm in diameter) and reticular veins (1 to 3 mm in diameter). Polidocanol works by damaging the cell lining of blood vessels, causing them to close and eventually be replaced by other types of tissue. <ref>Facts and Companies: </ref><ref>{{cite web |url=http://www.theodora.com/drugs/asclera_polidocanol_bioform_medical.html |title=Asclera Full Prescribing Information in Drug Reference Encyclopedia |format= |work= |accessdate=2010-04-11}}</ref> |
|
The FDA has approved polidocanol injections for the treatment of small varicose (less than 1 mm in diameter) and reticular veins (1 to 3 mm in diameter). Polidocanol works by damaging the cell lining of blood vessels, causing them to close and eventually be replaced by other types of tissue. <ref>Facts and Companies: </ref><ref>{{cite web |url=http://www.theodora.com/drugs/asclera_polidocanol_bioform_medical.html |title=Asclera Full Prescribing Information in Drug Reference Encyclopedia |format= |work= |accessdate=2010-04-11}}</ref> |
The FDA has approved polidocanol injections for the treatment of small varicose (less than 1 mm in diameter) and reticular veins (1 to 3 mm in diameter). Polidocanol works by damaging the cell lining of blood vessels, causing them to close and eventually be replaced by other types of tissue.