| Revision as of 10:03, 10 April 2011 editGrutness (talk | contribs)Autopatrolled, Administrators316,605 editsmNo edit summary← Previous edit | Revision as of 12:08, 30 April 2011 edit undoNono64 (talk | contribs)Autopatrolled, Pending changes reviewers, Rollbackers96,246 editsm chalconoidsNext edit → | ||
| Line 29: | Line 29: | ||
| }} | }} | ||
| }} | }} | ||
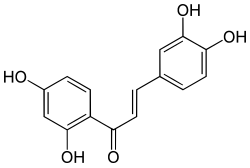
| '''Butein''' is a ]. It can be found in '']'' (or formerly ''Rhus verniciflua''). It has ], ] and ]s inhibitory effects<ref name="pmid18670102">{{cite journal |author=Lee EH, Song DG, Lee JY, Pan CH, Um BH, Jung SH |title=Inhibitory effect of the compounds isolated from Rhus verniciflua on aldose reductase and advanced glycation endproducts |journal=Biol. Pharm. Bull. |volume=31 |issue=8 |pages=1626–30 |year=2008 |month=August |pmid=18670102 |doi= 10.1248/bpb.31.1626|url=http://joi.jlc.jst.go.jp/JST.JSTAGE/bpb/31.1626?from=PubMed}}</ref>. It is also a ], a chemical compound having an effect on sirtuins, a group of enzymes that use NAD<sup>+</sup> to remove acetyl groups from proteins. | '''Butein''' is a ]. It can be found in '']'' (or formerly ''Rhus verniciflua''). It has ], ] and ]s inhibitory effects<ref name="pmid18670102">{{cite journal |author=Lee EH, Song DG, Lee JY, Pan CH, Um BH, Jung SH |title=Inhibitory effect of the compounds isolated from Rhus verniciflua on aldose reductase and advanced glycation endproducts |journal=Biol. Pharm. Bull. |volume=31 |issue=8 |pages=1626–30 |year=2008 |month=August |pmid=18670102 |doi= 10.1248/bpb.31.1626|url=http://joi.jlc.jst.go.jp/JST.JSTAGE/bpb/31.1626?from=PubMed}}</ref>. It is also a ], a chemical compound having an effect on sirtuins, a group of enzymes that use NAD<sup>+</sup> to remove acetyl groups from proteins. | ||
| ==References== | ==References== | ||
| {{reflist}} | {{reflist}} | ||
| {{ |
{{chalconoid}} | ||
| ] | ] | ||
| {{Natural-phenol-stub}} | {{Natural-phenol-stub}} | ||
| {{aromatic-stub}} | |||
Revision as of 12:08, 30 April 2011
 | |
| Names | |
|---|---|
| IUPAC name (E)-1-(2,4-dihydroxyphenyl)-3-(3,4-dihydroxyphenyl)prop-2-en-1-one | |
| Other names
2',3,4,4'-Tetrahydroxychalcone 2',4',3,4-Tetrahydroxychalcone 3,4,2',4'-Tetrahydroxychalcone 2′,4′,3,4-Tetrahydroxychalcone | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ECHA InfoCard | 100.006.963 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
SMILES
| |
| Properties | |
| Chemical formula | C15H12O5 |
| Molar mass | 272.25 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Butein is a chalconoid. It can be found in Toxicodendron vernicifluum (or formerly Rhus verniciflua). It has antioxidative, aldose reductase and advanced glycation endproducts inhibitory effects. It is also a sirtuin-activating compound, a chemical compound having an effect on sirtuins, a group of enzymes that use NAD to remove acetyl groups from proteins.
References
- Lee EH, Song DG, Lee JY, Pan CH, Um BH, Jung SH (2008). "Inhibitory effect of the compounds isolated from Rhus verniciflua on aldose reductase and advanced glycation endproducts". Biol. Pharm. Bull. 31 (8): 1626–30. doi:10.1248/bpb.31.1626. PMID 18670102.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
| Chalconoids and their glycosides | |
|---|---|
| Chalconoids | |
| Chalconoid glycosides | |
| Acylated chalconoids |
|
| O-methylated chalconoids |
|
| Flavokavains | |
| Synthetic | |