| Revision as of 18:42, 7 April 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (← Previous edit | Revision as of 17:18, 4 May 2011 edit undoCocuBot (talk | contribs)11,625 editsm r2.6.1) (robot Adding: no:DiazodinitrofenolNext edit → | ||
| Line 46: | Line 46: | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | ] | ||
| ] | ] | ||
Revision as of 17:18, 4 May 2011
| This article does not cite any sources. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Diazodinitrophenol" – news · newspapers · books · scholar · JSTOR (December 2009) (Learn how and when to remove this message) |
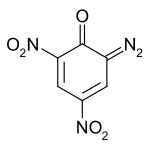 | |
| Names | |
|---|---|
| IUPAC name 6-Diazo-2,4-dinitrocyclohexa-2,4-dien-1-one | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Abbreviations | DDNP |
| ECHA InfoCard | 100.022.849 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
SMILES
| |
| Properties | |
| Chemical formula | C6H2N4O5 |
| Molar mass | 210.10 g/mol |
| Solubility in water | Insoluble |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Diazodinitrophenol (DDNP) is a yellowish brown powder. It is soluble in acetic acid, acetone, concentrated hydrochloric acid, and most non-polar solvents but is insoluble in water.
A solution of cold sodium hydroxide may be used to destroy it. DDNP may be desensitized by immersing it in water, as it does not react in water at normal temperature. It is less sensitive to impact but more powerful than mercury fulminate and lead azide. The sensitivity of DDNP to friction is much less than that of mercury fulminate, but it is approximately that of lead azide.
DDNP is used with other materials to form priming mixtures, particularly where a high sensitivity to flame or heat is desired. DDNP is often used as an initiating explosive in propellant primer devices and is a substitute for lead styphnate in what are termed "non-toxic" (lead free) priming explosive compositions.