| Revision as of 11:55, 18 April 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (← Previous edit | Revision as of 01:16, 31 July 2011 edit undoArcadian (talk | contribs)163,050 edits removed Category:Oncology; added Category:Carcinogenesis using HotCatNext edit → | ||
| Line 43: | Line 43: | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
Revision as of 01:16, 31 July 2011
| |
| Names | |
|---|---|
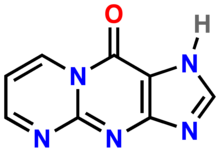
| IUPAC name pyrimidopurin-10(3H)-one | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
SMILES
| |
| Properties | |
| Chemical formula | C8H5N5O |
| Molar mass | 187.1582 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
M1G (pyrimidopurin-10(3H)-one) is a heterocyclic compound which is a by-product of base excision repair (BER) of a specific type of DNA adduct called M1dG. The M1dG adduct in turn is formed by a condensation reaction between guanosine nucleotides in DNA and either malondialdehyde or base propenal. If not repaired, these adducts are mutagenic and carcinogenic.
Malondialdehyde is an end product of lipid peroxidation while base propenal is a result of DNA peroxidation.
M1dG is the major endogenous DNA adduct in humans. M1dG adducts have been detected in cell DNA in liver, leucocytes, pancreas and breast in concentrations of 1-120 per 10 nucleotides. Detection and quantification of M1dG adducts in the body as measured by free M1G is a tool for detecting DNA damage that may lead to cancer. Free M1G is also biomarker for oxidative stress.
References
- ^ Marnett LJ (1999). "Lipid peroxidation-DNA damage by malondialdehyde". Mutat. Res. 424 (1–2): 83–95. doi:10.1016/S0027-5107(99)00010-X. PMID 10064852.
- ^ Seto H, Okuda T, Takesue T, Ikemura T (1983). "Reaction of Malonaldehyde with Nucleic Acid. I. Formation of Fluorescent Pyrimidopurin-10(3H)-one Nucleosides". Bulletin of the Chemical Society of Japan. 56 (6): 1799–1802. doi:10.1246/bcsj.56.1799.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Knutson CG, Akingbade D, Crews BC, Voehler M, Stec DF, Marnett LJ (2007). "Metabolism in vitro and in vivo of the DNA base adduct, M1G". Chem. Res. Toxicol. 20 (3): 550–7. doi:10.1021/tx600334x. PMID 17311424.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
External links
- pyrimido(1,2-a)purin-10(3H)-one at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- 3-(2'-deoxy-beta-D-erythro-pentofuranosyl)pyrimido(1,2-alpha)purin-10(3H)-one at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
This biochemistry article is a stub. You can help Misplaced Pages by expanding it. |