| Revision as of 21:24, 31 August 2011 editBogBot (talk | contribs)Bots53,132 edits populated new fields in {{drugbox}} and reordered per bot approval. Report errors and suggestions to User_talk:BogBot← Previous edit | Revision as of 21:36, 31 August 2011 edit undoCheMoBot (talk | contribs)Bots141,565 edits Updating {{drugbox}} (no changed fields - added verified revid - updated 'ChemSpiderID_Ref', 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEBI_Ref') per [[WP:CHEMVALID|Chem/Drugbox validationNext edit → | ||
| Line 1: | Line 1: | ||
| {{Drugbox | {{Drugbox | ||
| | verifiedrevid = |
| verifiedrevid = 442309649 | ||
| | image = Mertansine mab structure.svg | | image = Mertansine mab structure.svg | ||
| Line 33: | Line 33: | ||
| | ATC_suffix = | | ATC_suffix = | ||
| | PubChem = | | PubChem = | ||
| | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| | DrugBank = | | DrugBank = | ||
Revision as of 21:36, 31 August 2011
Pharmaceutical compound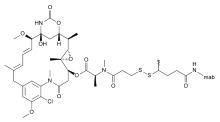 | |
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Humanized (from mouse) |
| Target | CD44 v6 |
| Clinical data | |
| ATC code |
|
| (verify) | |
Bivatuzumab mertansine is a combination of bivatuzumab, a humanized monoclonal antibody, and mertansine, a cytotoxic agent. It is designed for the treatment of squamous cell carcinoma.
References
- Tijink, BM; Buter, J; De Bree, R; Giaccone, G; Lang, MS; Staab, A; Leemans, CR; Van Dongen, GA (2006). "A phase I dose escalation study with anti-CD44v6 bivatuzumab mertansine in patients with incurable squamous cell carcinoma of the head and neck or esophagus". Clinical cancer research : an official journal of the American Association for Cancer Research. 12 (20 Pt 1): 6064–72. doi:10.1158/1078-0432.CCR-06-0910. PMID 17062682.
This monoclonal antibody–related article is a stub. You can help Misplaced Pages by expanding it. |