| Revision as of 10:50, 6 December 2011 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{drugbox}} taken from revid 464297937 of page Ketamine for the Chem/Drugbox validation project (updated: '').← Previous edit |
Revision as of 10:54, 6 December 2011 edit undoBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{drugbox}} taken from revid 464344515 of page Lefetamine for the Chem/Drugbox validation project (updated: 'CAS_number').Next edit → |
| Line 1: |
Line 1: |
|
{{ambox | text = This page contains a copy of the infobox ({{tl|drugbox}}) taken from revid of page ] with values updated to verified values.}} |
|
{{ambox | text = This page contains a copy of the infobox ({{tl|drugbox}}) taken from revid of page ] with values updated to verified values.}} |
|
{{drugbox |
|
{{Drugbox |
|
| verifiedrevid = 464192226 |
|
| verifiedrevid = 451642269 |
|
| IUPAC_name = (''RS'')-2-(2-Chlorophenyl)-2-(methylamino)cyclohexanone |
|
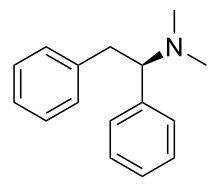
| IUPAC_name = (1''R'')-''N'',''N''-dimethyl-1,2-diphenylethanamine |
|
| image = Ketamine.svg |
|
| image = Lefetamine.svg |
|
|
| image2 = Lefetamine3d.png |
|
| width = 150 |
|
|
⚫ |
| width2 = 160 |
|
| image2 = Ketamine.pdb.gif |
|
| ⚫ |
| width2 = 200 |
|
|
| imagename = 1 : 1 mixture (racemate) |
|
|
| drug_name = Ketamine |
|
|
|
|
|
|
<!--Clinical data--> |
|
<!--Clinical data--> |
|
|
| tradename = |
|
| Drugs.com = {{drugs.com|CDI|ketamine}} |
|
|
⚫ |
| pregnancy_category = |
|
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
|
|
|
| legal_status = ] (]) |
|
| pregnancy_US = <!-- A / B / C / D / X --> |
|
|
|
| routes_of_administration = ] |
| ⚫ |
| pregnancy_category = B |
|
|
| legal_AU = S8 |
|
|
| legal_CA = Schedule I |
|
|
| legal_UK = Class C |
|
|
| legal_US = Schedule III |
|
|
| routes_of_administration = ], ], ]d, oral, ] |
|
|
|
|
|
|
<!--Pharmacokinetic data--> |
|
<!--Pharmacokinetic data--> |
|
|
| bioavailability = |
|
| metabolism = Hepatic, primarily by CYP3A4<ref>{{Cite journal|author=Hijazi Y, Boulieu R|title=Contribution of CYP3A4, CYP2B6, and CYP2C9 isoforms to N-demethylation of ketamine in human liver microsomes|journal=Drug Metabolism and Disposition|volume=30|issue=7|pages=853–8|year=2002|month=July|pmid=12065445|doi=10.1124/dmd.30.7.853}}</ref> |
|
|
|
| metabolism = |
|
| elimination_half-life = 2.5–3 hours. |
|
| elimination_half-life = |
|
| excretion = renal (>90%) |
|
| excretion = |
|
|
|
|
|
<!--Identifiers--> |
|
<!--Identifiers--> |
|
| CASNo_Ref = {{cascite|correct|CAS}} |
|
|
| CAS_number_Ref = {{cascite|correct|??}} |
|
| CAS_number_Ref = {{cascite|correct|??}} |
|
| CAS_number = 6740-88-1 |
|
| CAS_number = <!-- blanked - oldvalue: 7262-75-1 --> |
|
| ATC_prefix = N01 |
|
| ATC_prefix = none |
|
| ATC_suffix = AX03 |
|
| ATC_suffix = |
|
⚫ |
| PubChem = 443970 |
|
| ATC_supplemental = {{ATC|N01|AX14}} |
|
|
| ChEBI_Ref = {{ebicite|correct|EBI}} |
|
|
| ChEBI = 6121 |
|
| ⚫ |
| PubChem = 3821 |
|
|
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|
|
| DrugBank = DB01221 |
|
|
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|
| ChemSpiderID = 3689 |
|
| ChemSpiderID = 392017 |
|
| UNII_Ref = {{fdacite|correct|FDA}} |
|
| UNII_Ref = {{fdacite|correct|FDA}} |
|
| UNII = 690G0D6V8H |
|
| UNII = 4J9726V5Y9 |
|
| KEGG_Ref = {{keggcite|correct|kegg}} |
|
|
| KEGG = D08098 |
|
|
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
|
|
| ChEMBL = 742 |
|
|
|
|
|
|
<!--Chemical data--> |
|
<!--Chemical data--> |
|
| C=13 | H=16 | Cl=1 | N=1 | O=1 |
|
| C=16 | H=19 | N=1 |
|
| molecular_weight = 237.725 g/mol |
|
| molecular_weight = 225.329 g/mol |
|
| smiles = CNC1(CCCCC1=O)c2ccccc2Cl |
|
| smiles = N(C)((c1ccccc1)Cc2ccccc2)C |
|
| InChI = 1/C13H16ClNO/c1-15-13(9-5-4-8-12(13)16)10-6-2-3-7-11(10)14/h2-3,6-7,15H,4-5,8-9H2,1H3 |
|
| InChI = 1/C16H19N/c1-17(2)16(15-11-7-4-8-12-15)13-14-9-5-3-6-10-14/h3-12,16H,13H2,1-2H3/t16-/m1/s1 |
|
|
| InChIKey = YEJZJVJJPVZXGX-MRXNPFEDBZ |
|
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChI = 1S/C13H16ClNO/c1-15-13(9-5-4-8-12(13)16)10-6-2-3-7-11(10)14/h2-3,6-7,15H,4-5,8-9H2,1H3 |
|
| StdInChI = 1S/C16H19N/c1-17(2)16(15-11-7-4-8-12-15)13-14-9-5-3-6-10-14/h3-12,16H,13H2,1-2H3/t16-/m1/s1 |
|
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChIKey = YQEZLKZALYSWHR-UHFFFAOYSA-N |
|
| StdInChIKey = YEJZJVJJPVZXGX-MRXNPFEDSA-N |
|
| melting_point = 262 |
|
|
}} |
|
}} |