| Revision as of 15:53, 6 December 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report errors or [[u...← Previous edit | Revision as of 10:44, 17 December 2011 edit undoNotWith (talk | contribs)61,905 edits Category:AldehydesNext edit → | ||
| Line 44: | Line 44: | ||
| '''Viniferal''' is a ] with an ] group found in '']'' (grapevine).<ref>{{cite journal | doi = 10.1016/0040-4020(96)00543-1 | title = Absolute structures of new hydroxystilbenoids, vitisin C and viniferal, from Vitis vinifera 'Kyohou' | year = 1996 | last1 = Ito | first1 = J | journal = Tetrahedron | volume = 52 | issue = 30 | pages = 9991}}</ref> | '''Viniferal''' is a ] with an ] group found in '']'' (grapevine).<ref>{{cite journal | doi = 10.1016/0040-4020(96)00543-1 | title = Absolute structures of new hydroxystilbenoids, vitisin C and viniferal, from Vitis vinifera 'Kyohou' | year = 1996 | last1 = Ito | first1 = J | journal = Tetrahedron | volume = 52 | issue = 30 | pages = 9991}}</ref> | ||
| ==References== | == References == | ||
| {{reflist}} | {{reflist}} | ||
| ==External links== | == External links == | ||
| * | * | ||
| Line 53: | Line 53: | ||
| ] | ] | ||
| ] | |||
| ] | ] | ||
Revision as of 10:44, 17 December 2011
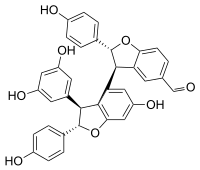
| |
| Names | |
|---|---|
| IUPAC name (2R,2′S,3R,3′S)-3′-(3,5-Dihydroxyphenyl)-6′-hydroxy-2,2′-bis(4-hydroxyphenyl)-2,2′,3,3′-tetrahydro--5-carbaldehyde | |
| Other names (-)-Viniferal | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C35H26O8 |
| Molar mass | 574.585 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Viniferal is a hydroxystilbenoid with an aldehyde group found in Vitis vinifera (grapevine).
References
- Ito, J (1996). "Absolute structures of new hydroxystilbenoids, vitisin C and viniferal, from Vitis vinifera 'Kyohou'". Tetrahedron. 52 (30): 9991. doi:10.1016/0040-4020(96)00543-1.
External links
| Type of stilbenoids (molecules with a C6-C2-C6 backbone) | |
|---|---|
| Types |
|