| Revision as of 23:15, 10 February 2012 editAniRaptor2001 (talk | contribs)Extended confirmed users, Pending changes reviewers6,327 edits bolded street names← Previous edit | Revision as of 23:18, 10 February 2012 edit undoAniRaptor2001 (talk | contribs)Extended confirmed users, Pending changes reviewers6,327 edits added sourceNext edit → | ||
| Line 43: | Line 43: | ||
| }} | }} | ||
| '''2-Diphenylmethylpyrrolidine''' ('''Desoxy-D2PM'''), also known as '''2-benzhydrylpyrrolidine''', is a ] ]. It is the 4-dehydroxylated ] of ] (D2PM), and is also similar in structure to ] (2-DPMP), both of which act as ]s (NDRIs). Like D2PM and 2-DPMP, Desoxy-D2PM is sold as a ], and has been used in the manufacture of ]s. It has been marketed under the names '''A3A New Generation''', '''A3A Methano''', and '''Green Powder''', and has been reported to cause hallucinations, violent behavior, dilated pupils, tachycardia, and high blood pressure.<ref>http://www.ncbi.nlm.nih.gov/pubmed/22055832</ref><ref>http://www.droganews.it/pubdownload.php?id=2472</ref> | '''2-Diphenylmethylpyrrolidine''' ('''Desoxy-D2PM'''), also known as '''2-benzhydrylpyrrolidine''', is a ] ]. It is the 4-dehydroxylated ] of ] (D2PM), and is also similar in structure to ] (2-DPMP), both of which act as ]s (NDRIs). Like D2PM and 2-DPMP, Desoxy-D2PM is sold as a ], and has been used in the manufacture of ]s. It has been marketed under the names '''A3A New Generation''', '''A3A Methano''', and '''Green Powder''', and has been reported to cause hallucinations, violent behavior, dilated pupils, tachycardia, and high blood pressure.<ref>http://www.ncbi.nlm.nih.gov/pubmed/22055832</ref><ref>http://www.droganews.it/pubdownload.php?id=2472</ref><ref>http://ewsd.wiv-isp.be/Other%20information%20on%20new%20psychoactive%20substances/Desoxy-D2PM/Report_UK_Dec%202010_Green%20Powder%20called%20A3A%20Methano.pdf</ref> | ||
| Desoxy-D2PM has two ]s which are used industrially in their purified form for resolution of chiral reagents during chemical synthesis.<ref>Bertelsen S, Halland N, Bachmann S, Marigo M, Braunton A, Jørgensen KA. Organocatalytic asymmetric α-bromination of aldehydes and ketones. ''Chem. Commun.'' 2005, 4821-4823{{doi|10.1039/b509366j}}</ref> | Desoxy-D2PM has two ]s which are used industrially in their purified form for resolution of chiral reagents during chemical synthesis.<ref>Bertelsen S, Halland N, Bachmann S, Marigo M, Braunton A, Jørgensen KA. Organocatalytic asymmetric α-bromination of aldehydes and ketones. ''Chem. Commun.'' 2005, 4821-4823{{doi|10.1039/b509366j}}</ref> | ||
Revision as of 23:18, 10 February 2012
Pharmaceutical compound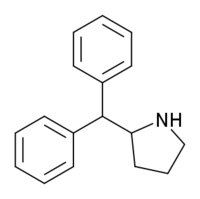 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.162.251 |
| Chemical and physical data | |
| Formula | C17H19N |
| Molar mass | 237.339 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
2-Diphenylmethylpyrrolidine (Desoxy-D2PM), also known as 2-benzhydrylpyrrolidine, is a stimulant psychoactive drug. It is the 4-dehydroxylated structural analogue of diphenylprolinol (D2PM), and is also similar in structure to desoxypipradrol (2-DPMP), both of which act as norepinephrine-dopamine reuptake inhibitors (NDRIs). Like D2PM and 2-DPMP, Desoxy-D2PM is sold as a designer drug, and has been used in the manufacture of legal highs. It has been marketed under the names A3A New Generation, A3A Methano, and Green Powder, and has been reported to cause hallucinations, violent behavior, dilated pupils, tachycardia, and high blood pressure.
Desoxy-D2PM has two enantiomers which are used industrially in their purified form for resolution of chiral reagents during chemical synthesis.
References
- http://www.ncbi.nlm.nih.gov/pubmed/22055832
- http://www.droganews.it/pubdownload.php?id=2472
- http://ewsd.wiv-isp.be/Other%20information%20on%20new%20psychoactive%20substances/Desoxy-D2PM/Report_UK_Dec%202010_Green%20Powder%20called%20A3A%20Methano.pdf
- Bertelsen S, Halland N, Bachmann S, Marigo M, Braunton A, Jørgensen KA. Organocatalytic asymmetric α-bromination of aldehydes and ketones. Chem. Commun. 2005, 4821-4823doi:10.1039/b509366j
This drug article relating to the nervous system is a stub. You can help Misplaced Pages by expanding it. |