| Revision as of 12:33, 15 February 2012 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 473697878 of page Allyl_alcohol for the Chem/Drugbox validation project (updated: '').← Previous edit |
Revision as of 12:33, 15 February 2012 edit undoBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 473697840 of page Sulfamic_acid for the Chem/Drugbox validation project (updated: '').Next edit → |
| Line 1: |
Line 1: |
|
{{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} |
|
{{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} |
|
{{chembox |
|
{{chembox |
|
|
| Verifiedfields = changed |
|
| verifiedrevid = 443376202 |
|
| verifiedrevid = 417637524 |
|
| ImageFile = Allyl-alcohol.png |
|
|
|
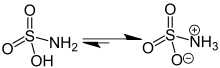
| Name = Sulfamic acid |
|
| ImageName = Skeletal formula |
|
|
|
| ImageFile = Zwitterion Structural Formulae V.1.svg |
| ⚫ |
| ImageFile1 = Allyl-alcohol-3D-balls-2.png |
|
|
|
| ImageSize = 220px |
| ⚫ |
| ImageName1 = Ball-and-stick model |
|
|
|
| ImageName = Tautomerism of sulfamic acid |
|
| IUPACName = Prop-2-en-1-ol, Allyl alcohol |
|
|
⚫ |
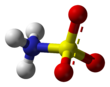
| ImageFileL1 = Sulfamic-acid-3D-balls.png |
|
|
| ImageSizeL1 = 110px |
|
|
| ImageNameL1 = Ball-and-stick model of the canonical neutral form |
|
|
| ImageFileR1 = Sulfamic-acid-zwitterion-3D-balls.png |
|
|
| ImageSizeR1 = 110px |
|
⚫ |
| ImageNameR1 = Ball-and-stick model of the zwitterionic form |
|
|
| IUPACName = Sulfamic acid |
|
| Section1 = {{Chembox Identifiers |
|
| Section1 = {{Chembox Identifiers |
|
| KEGG_Ref = {{keggcite|correct|kegg}} |
|
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|
| KEGG = C02001 |
|
| ChemSpiderID = 5767 |
|
|
| PubChem = 5987 |
|
| InChIKey = XXROGKLTLUQVRX-UHFFFAOYAC |
|
|
⚫ |
| InChI = 1/H3NO3S/c1-5(2,3)4/h(H3,1,2,3,4) |
|
|
| InChIKey = IIACRCGMVDHOTQ-UHFFFAOYAK |
|
⚫ |
| ChEBI_Ref = {{ebicite|changed|EBI}} |
|
|
| ChEBI = 9330 |
|
⚫ |
| SMILES = O=S(=O)(O)N |
|
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
|
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
|
| ChEMBL = 234926 |
|
| ChEMBL = 68253 |
|
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChI = 1S/C3H6O/c1-2-3-4/h2,4H,1,3H2 |
|
| StdInChI = 1S/H3NO3S/c1-5(2,3)4/h(H3,1,2,3,4) |
|
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChIKey = XXROGKLTLUQVRX-UHFFFAOYSA-N |
|
| StdInChIKey = IIACRCGMVDHOTQ-UHFFFAOYSA-N |
|
| CASNo_Ref = {{cascite|correct|CAS}} |
|
| CASNo = 5329-14-6 |
|
|
| CASNo_Ref = {{cascite|correct|CAS}} |
|
|
| UNNumber = 2967 |
|
| CASNo=107-18-6 |
|
|
|
| EINECS = 226-218-8 |
| ⚫ |
| ChEBI_Ref = {{ebicite|correct|EBI}} |
|
|
| ChEBI = 16605 |
|
| RTECS = WO5950000 |
|
|
}} |
| ⚫ |
| SMILES = C=CCO |
|
|
⚫ |
| Section2 = {{Chembox Properties |
|
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|
|
|
| Formula = H<sub>3</sub>NSO<sub>3</sub> |
|
| ChemSpiderID=13872989 |
|
|
|
| MolarMass = 97.10 g/mol |
| ⚫ |
| InChI=1/C3H6O/c1-2-3-4/h2,4H,1,3H2}} |
|
|
⚫ |
| MeltingPt = 205 °C decomp. |
| ⚫ |
|Section2 = {{Chembox Properties |
|
|
|
| Density = 2.15 g/cm<sup>3</sup> |
|
| C = 3 | H = 6 | O = 1 |
|
|
|
| Solubility = moderate, with slow hydrolysis |
|
| Density=0.854 g/ml |
|
|
|
| pKa = 1.0<ref>{{cite doi|10.1039/JR9600004236}}</ref> |
| ⚫ |
| MeltingPt=−129 °C |
|
|
|
}} |
|
| BoilingPt=97 °C |
|
|
⚫ |
| Section7 = {{Chembox Hazards |
|
| Solubility=Miscible |
|
|
|
| ExternalMSDS = |
|
| Solvent=Water |
|
|
⚫ |
| EUIndex = 016-026-00-0 |
|
|
| EUClass = Irritant ('''Xi''') |
|
|
| RPhrases = {{R36/38}} {{R52/53}} |
|
⚫ |
| SPhrases = {{S2}} {{S26}} {{S28}} {{S61}} |
|
⚫ |
| NFPA-H = |
|
⚫ |
| NFPA-F = |
|
⚫ |
| NFPA-R = |
|
|
| NFPA-O = |
|
⚫ |
| FlashPt = |
|
|
}} |
|
|
| Section8 = {{Chembox Related |
|
|
| OtherAnions = |
|
|
| OtherCations = ] |
|
|
| OtherFunctn = |
|
|
| Function = |
|
|
| OtherCpds = |
|
}} |
|
}} |
| ⚫ |
|Section3={{Chembox Hazards |
|
|
| EUClass=Toxic ('''T''')<br />Dangerous for<br />the environment ('''N''') |
|
| ⚫ |
| EUIndex=603-015-00-6 |
|
|
| ExternalMSDS = |
|
| ⚫ |
| NFPA-H=3 |
|
| ⚫ |
| NFPA-F=3 |
|
| ⚫ |
| NFPA-R=0 |
|
|
| RPhrases={{R10}}, {{R23/24/25}},<br />{{R36/37/38}}, {{R50}} |
|
| ⚫ |
| SPhrases={{S1/2}}, {{S36/37/39}},<br />{{S38}}, {{S45}}, {{S61}} |
|
| ⚫ |
| FlashPt=21 °C |
|
|
| Autoignition=378 °C |
|
|
| ExploLimits=2.5–18.0%}} |
|
|
}} |
|
}} |