| Revision as of 12:34, 15 February 2012 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 476973632 of page Sulfur_hexafluoride for the Chem/Drugbox validation project (updated: 'UNII', 'KEGG').← Previous edit | Revision as of 12:34, 15 February 2012 edit undoBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 473657671 of page Gallium_nitride for the Chem/Drugbox validation project (updated: '').Next edit → | ||
| Line 1: | Line 1: | ||
| {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | ||
| {{ |
{{chembox | ||
| | |
| Watchedfields = changed | ||
| | verifiedrevid = |
| verifiedrevid = 450764160 | ||
| | Name = Gallium nitride | |||
| | ImageFileL1 = Sulfur-hexafluoride-2D-dimensions.png | |||
| | ImageFile = GaNcrystal.jpg | |||
| | ImageFileL1_Ref = {{chemboximage|correct|??}} | |||
| | ImageFile2 = Wurtzite polyhedra.png | |||
| | ImageSizeL1 = 121 | |||
| | IUPACName = Gallium nitride | |||
| | ImageNameL1 = Skeletal formula of sulfur hexafluoride with assorted dimensions | |||
| | OtherNames = | |||
| | ImageFileR1 = Sulfur-hexafluoride-3D-vdW.png | |||
| | Name = | |||
| | ImageFileR1_Ref = {{chemboximage|correct|??}} | |||
| | ImageSizeR1 = 121 | |||
| | ImageNameR1 = Spacefill model of sulfur hexafluoride | |||
| | ImageFile2 = Sulfur-hexafluoride-3D-balls.png | |||
| | ImageFile2_Ref = {{chemboximage|correct|??}} | |||
| | ImageSize2 = 121 | |||
| | ImageName2 = Ball and stick model of sulfur hexafluoride | |||
| | IUPACName = Sulfur hexafluoride | |||
| | SystematicName = Hexafluoro-λ<sup>6</sup>-sulfane<ref>{{Cite web|title = Sulfur Hexafluoride - PubChem Public Chemical Database|url = http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=17358|work = The PubChem Project|location = USA|publisher = National Center for Biotechnology Information}}</ref> | |||
| | OtherNames = Elagas<br /> | |||
| Esaflon<br /> | |||
| Sulfur(VI) fluoride<br /> | |||
| Sulfuric fluoride | |||
| | Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | CASNo = 2551-62-4 | |||
| | ChemSpiderID = 105057 | |||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | InChI = 1/Ga.N/rGaN/c1-2 | |||
| | PubChem = 17358 | |||
| | SMILES = #N | |||
| | PubChem_Ref = {{Pubchemcite}} | |||
| | InChIKey = JMASRVWKEDWRBT-MDMVGGKAAI | |||
| | ChemSpiderID = 16425 | |||
| | |
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/Ga.N | |||
| | UNII = <!-- blanked - oldvalue: WS7LR3I1D6 --> | |||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | UNII_Ref = {{fdacite|changed|FDA}} | |||
| | StdInChIKey = JMASRVWKEDWRBT-UHFFFAOYSA-N | |||
| | EINECS = 219-854-2 | |||
| | |
| CASNo = 25617-97-4 | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | KEGG = <!-- blanked - oldvalue: D05962 --> | |||
| | PubChem = 117559 | |||
| | KEGG_Ref = {{keggcite|changed|kegg}} | |||
| | RTECS = LW9640000 | |||
| | MeSHName = Sulfur+hexafluoride | |||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | ChEBI = 30496 | |||
| | RTECS = WS4900000 | |||
| | ATCCode_prefix = V08 | |||
| | ATCCode_suffix = DA05 | |||
| | Gmelin = 2752 | |||
| | SMILES = FS(F)(F)(F)(F)F | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChI = 1S/F6S/c1-7(2,3,4,5)6 | |||
| | StdInChIKey = SFZCNBIFKDRMGX-UHFFFAOYSA-N | |||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| }} | }} | ||
| | Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| | |
| Formula = GaN | ||
| | MolarMass = 83.73 g/mol | |||
| | S = 1 | |||
| | Appearance = yellow powder | |||
| | ExactMass = 145.962489920 g mol<sup>-1</sup> | |||
| | Density = 6.15 g/cm<sup>3</sup> | |||
| | Appearance = Colorless, odorless gas | |||
| | |
| Solubility = Reacts. | ||
| | MeltingPt = >2500°C<ref name=melting>{{cite journal| author = T. Harafuji and J. Kawamura|title = Molecular dynamics simulation for evaluating melting point of wurtzite-type GaN crystal| doi = 10.1063/1.1772878|journal = Appl. Phys.|volume = 96| page = 2501|year = 2004| issue = 5}}</ref> | |||
| | BoilingPtK = 209 | |||
| | BoilingPt = | |||
| | VaporPressure = 2.9 kPa (at 21.1°C) | |||
| | pKb = | |||
| | BandGap = 3.4 eV (300 K, direct) | |||
| | ElectronMobility = 440 cm<sup>2</sup>/(V·s) (300 K) | |||
| | ThermalConductivity = 2.3 W/(cm·K) (300 K) <ref>Mion, Christian. "Investigation of the Thermal Properties of Gallium | |||
| Nitride Using the Three Omega Technique." Diss. North Carolina State University. Raleigh, 2005. Web, Aug 12, 2011. http://repository.lib.ncsu.edu/ir/bitstream/1840.16/5418/1/etd.pdf.</ref> | |||
| | RefractIndex = 2.429 | |||
| }} | }} | ||
| | Section3 = {{Chembox Structure | | Section3 = {{Chembox Structure | ||
| | |
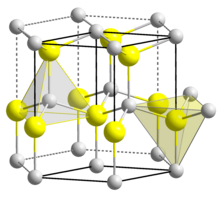
| CrystalStruct = ] | ||
| | |
| SpaceGroup = ''C''<sub>6v</sub><sup>4</sup>-''P''6<sub>3</sub>''mc'' | ||
| | |
| Coordination = Tetrahedral | ||
| | LattConst_a = 3.186 Å | |||
| | MolShape = Octahedral | |||
| | LattConst_c = 5.186 Å <ref>Bougrov V., Levinshtein M.E., Rumyantsev S.L., Zubrilov A., in ''Properties of Advanced Semiconductor Materials GaN, AlN, InN, BN, SiC, SiGe''. Eds. Levinshtein M.E., Rumyantsev S.L., Shur M.S., John Wiley & Sons, Inc., New York, 2001, 1–30</ref> | |||
| | Dipole = 0 D | |||
| }} | }} | ||
| | |
| Section7 = {{Chembox Hazards | ||
| | EUIndex = Not listed | |||
| | DeltaHf = −1209 kJ·mol<sup>−1</sup><ref name=b1>{{cite book| author = Zumdahl, Steven S.|title =Chemical Principles 6th Ed.| publisher = Houghton Mifflin Company| year = 2009| isbn = 061894690X|page=A23}}</ref> | |||
| | RPhrases = | |||
| | Entropy = 292 J·mol<sup>−1</sup>·K<sup>−1</sup><ref name=b1/> | |||
| | SPhrases = | |||
| }} | |||
| | FlashPt = Non-flammable. | |||
| | Section5 = {{Chembox Hazards | |||
| }} | |||
| | ExternalMSDS = | |||
| | |
| Section8 = {{Chembox Related | ||
| | OtherAnions = ]<br/>]<br/>] | |||
| | NFPA-H = 0 | |||
| | OtherCations = ]<br/>]<br/>] | |||
| | NFPA-F = 0 | |||
| | |
| Function = | ||
| | OtherFunctn = | |||
| | OtherCpds = ]<br/>]<br/>]<br/>]<br/>] | |||
| }} | }} | ||
| | Section6 = {{Chembox Related | |||
| | OtherCations = | |||
| | Function = sulfur fluorides | |||
| | OtherFunctn = ]<br /> | |||
| ] | |||
| | OtherCpds = ]<br /> | |||
| ]<br /> | |||
| ] | |||
| }} | |||
| }} | }} | ||
Revision as of 12:34, 15 February 2012
| This page contains a copy of the infobox ({{chembox}}) taken from revid 473657671 of page Gallium_nitride with values updated to verified values. |

| |
| |
| Names | |
|---|---|
| IUPAC name Gallium nitride | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| RTECS number |
|
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | GaN |
| Molar mass | 83.73 g/mol |
| Appearance | yellow powder |
| Density | 6.15 g/cm |
| Melting point | >2500°C |
| Solubility in water | Reacts. |
| Band gap | 3.4 eV (300 K, direct) |
| Electron mobility | 440 cm/(V·s) (300 K) |
| Thermal conductivity | 2.3 W/(cm·K) (300 K) |
| Refractive index (nD) | 2.429 |
| Structure | |
| Crystal structure | Wurtzite |
| Space group | C6v-P63mc |
| Lattice constant | a = 3.186 Å, c = 5.186 Å |
| Coordination geometry | Tetrahedral |
| Hazards | |
| Flash point | Non-flammable. |
| Related compounds | |
| Other anions | Gallium phosphide Gallium arsenide Gallium antimonide |
| Other cations | Boron nitride Aluminium nitride Indium nitride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
- T. Harafuji and J. Kawamura (2004). "Molecular dynamics simulation for evaluating melting point of wurtzite-type GaN crystal". Appl. Phys. 96 (5): 2501. doi:10.1063/1.1772878.
- Mion, Christian. "Investigation of the Thermal Properties of Gallium Nitride Using the Three Omega Technique." Diss. North Carolina State University. Raleigh, 2005. Web, Aug 12, 2011. http://repository.lib.ncsu.edu/ir/bitstream/1840.16/5418/1/etd.pdf.
- Bougrov V., Levinshtein M.E., Rumyantsev S.L., Zubrilov A., in Properties of Advanced Semiconductor Materials GaN, AlN, InN, BN, SiC, SiGe. Eds. Levinshtein M.E., Rumyantsev S.L., Shur M.S., John Wiley & Sons, Inc., New York, 2001, 1–30