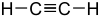| Revision as of 01:06, 27 February 2013 editAddbot (talk | contribs)Bots2,838,809 editsm Bot: Migrating 8 interwiki links, now provided by Wikidata on d:q485014 (Report Errors)← Previous edit | Revision as of 09:36, 2 July 2013 edit undoGklambauer (talk | contribs)53 editsm Added references and links.Next edit → | ||
| Line 2: | Line 2: | ||
| : ''For other uses of analog, see ].'' | : ''For other uses of analog, see ].'' | ||
| In ], a '''structural analog''' ('''structural analogue'''), also known as '''chemical analog''' or simply '''analog''', is a ] having a structure similar to that of another one, but differing from it in respect of a certain component. |
In ], a '''structural analog''' ('''structural analogue'''), also known as '''chemical analog''' or simply '''analog''', is a ] having a structure similar to that of another one, but differing from it in respect of a certain component<ref name="Willet1998">{{cite journal | author =Willett, Peter, Barnard, John M. and Downs, Geoffry M. | title = Chemical Similarity Searching | journal = Journal of Chemical Information and Computer Science | year = 1998 | volume = 38 | pages = 983−996}} </ref><ref name="Johnson1990">{{cite book | author = A. M. Johnson, G. M. Maggiora | title = Concepts and Applications of Molecular Similarity | publisher = John Willey & Sons | location = New York | year = 1990 | isbn = 0-471-62175-7}}</ref><ref name="Nikolova2003">{{cite journal | doi = 10.1002/qsar.200330831 | author = N. Nikolova, J. Jaworska | title = Approaches to Measure Chemical Similarity - a Review | journal = QSAR & Combinatorial Science | year = 2003 | volume = 22 | issue = 9-10 | pages = 1006–1026}}</ref>. | ||
| It can differ in one or more ]s, ]s, or substructures, which are replaced with other atoms, groups, or substructures. A structural analog can be imagined to be formed, at least theoretically, from the other compound. | |||
| Despite a high chemical similarity, structural analogs are not necessarily ]s and can have very different physical, chemical, biochemical, or pharmacological properties. | |||
| Despite a high chemical similarity, structural analogs are not necessarily ]s and can have very different physical, chemical, biochemical, or pharmacological properties<ref name="Martin2002">{{cite journal | author = Martin, Yvonne C., Kofron, James L. and Traphagen, Linda M. | title = Do Structurally Similar Molecules Have Similar Biological Activity? | journal = Journal of Medicinal Chemistry | year = 2002 | volume = 45(19) | pages =4350-4358}} </ref>. | |||
| In ] large series of structural analogs of an initial ] are created and tested as part of a ] study. | |||
| In ] either a large series of structural analogs of an initial ] are created and tested as part of a ] study<ref name="Schnecke2006">{{cite journal | author = Schnecke, Volker and Boström, Jonas | title = Computational chemistry-driven decision making in lead generation | journal = Drug Discovery Today | year = 2006 | volume = 11(1-2) | pages = 43-50}} </ref> or a database is ] for structural analogs of a ]<ref name="pmid18600572">{{cite journal | author = Rester, Ulrich | title = From virtuality to reality - Virtual screening in lead discovery and lead optimization: A medicinal chemistry perspective | journal = Current Opinion in Drug Discovery and Development | volume = 11 | issue = 4 | pages = 559–68 | year = 2008 | pmid = 18600572}}</ref>. | |||
| == Examples == | == Examples == | ||
| Line 53: | Line 55: | ||
| {{Physical-chemistry-stub}} | {{Physical-chemistry-stub}} | ||
| == References == | |||
| {{Reflist}} | |||
| == External links == | |||
| * — a free web-service for finding structural analogs in ]. | |||
Revision as of 09:36, 2 July 2013
| This article does not cite any sources. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Structural analog" – news · newspapers · books · scholar · JSTOR (December 2009) (Learn how and when to remove this message) |
- For other uses of analog, see Analog (disambiguation).
In chemistry, a structural analog (structural analogue), also known as chemical analog or simply analog, is a compound having a structure similar to that of another one, but differing from it in respect of a certain component.
It can differ in one or more atoms, functional groups, or substructures, which are replaced with other atoms, groups, or substructures. A structural analog can be imagined to be formed, at least theoretically, from the other compound.
Despite a high chemical similarity, structural analogs are not necessarily functional analogs and can have very different physical, chemical, biochemical, or pharmacological properties.
In drug development either a large series of structural analogs of an initial lead compound are created and tested as part of a structure-activity relationship study or a database is screened for structural analogs of a lead compound.
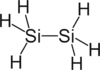
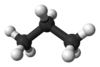
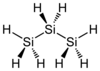
Examples
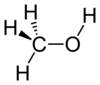
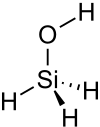
| Carbon-Based | Silicon-Based |
|---|---|
 |
 |
 |
 |
 |
 |
 |
 |
See also
- Derivative (chemistry)
- Homolog, a compound of a series differing only by repeated units
- Functional analog, compounds with similar physical, chemical, biochemical, or pharmacological properties
- Transition state analog
This physical chemistry-related article is a stub. You can help Misplaced Pages by expanding it. |
References
- Willett, Peter, Barnard, John M. and Downs, Geoffry M. (1998). "Chemical Similarity Searching". Journal of Chemical Information and Computer Science. 38: 983−996.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - A. M. Johnson, G. M. Maggiora (1990). Concepts and Applications of Molecular Similarity. New York: John Willey & Sons. ISBN 0-471-62175-7.
- N. Nikolova, J. Jaworska (2003). "Approaches to Measure Chemical Similarity - a Review". QSAR & Combinatorial Science. 22 (9–10): 1006–1026. doi:10.1002/qsar.200330831.
- Martin, Yvonne C., Kofron, James L. and Traphagen, Linda M. (2002). "Do Structurally Similar Molecules Have Similar Biological Activity?". Journal of Medicinal Chemistry. 45(19): 4350–4358.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Schnecke, Volker and Boström, Jonas (2006). "Computational chemistry-driven decision making in lead generation". Drug Discovery Today. 11(1-2): 43–50.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Rester, Ulrich (2008). "From virtuality to reality - Virtual screening in lead discovery and lead optimization: A medicinal chemistry perspective". Current Opinion in Drug Discovery and Development. 11 (4): 559–68. PMID 18600572.
External links
- Analoging in ChEMBL — a free web-service for finding structural analogs in ChEMBL.