| Revision as of 01:16, 16 December 2014 editQuimicabrasil (talk | contribs)7 editsNo edit summary← Previous edit | Revision as of 02:10, 16 December 2014 edit undoQuimicabrasil (talk | contribs)7 editsNo edit summaryNext edit → | ||
| Line 6: | Line 6: | ||
| | ImageSize = | | ImageSize = | ||
| | IUPACName = Lead 2,4,6-trinitrobenzene-1,3-diolate | | IUPACName = Lead 2,4,6-trinitrobenzene-1,3-diolate | ||
| | OtherNames = {{unbulleted list|Lead 2,4,6-trinitro-m-phenylene dioxide|1,3-Benzenediol, 2,4,6-trinitro-, lead(2+) salt (1:1)|Lead tricinate|Lead trinitroresorcinate| Tricinat}}<ref>http://echa.europa.eu/documents/10162/13638/svhc_supdoc_lead_styphnate_en.pdf</ref> | | OtherNames = {{unbulleted list|Lead 2,4,6-trinitro-m-phenylene dioxide|1,3-Benzenediol, 2,4,6-trinitro-, lead(2+) salt (1:1)|Lead tricinate|Lead trinitroresorcinate| Tricinat}}<ref>ECHA, European Chemicals Agency http://echa.europa.eu/documents/10162/13638/svhc_supdoc_lead_styphnate_en.pdf</ref> | ||
| |Section1={{Chembox Identifiers | |Section1={{Chembox Identifiers | ||
| Line 56: | Line 56: | ||
| :<math>C_{6}HN_{3}O_{8}Mg(H_{2}O) + Pb(CH_{3}COO)_{2} \longrightarrow C_{6}HN_{3}O_{8}Pb(H_{2}O) + Mg(CH_{3}COO)_{2}</math> | :<math>C_{6}HN_{3}O_{8}Mg(H_{2}O) + Pb(CH_{3}COO)_{2} \longrightarrow C_{6}HN_{3}O_{8}Pb(H_{2}O) + Mg(CH_{3}COO)_{2}</math> | ||
| Lead styphnate can be produced by the reaction of the magnesium styphnate salt with lead acetate. The presence of agents can promote the formation of specific crystalline forms. |
Lead styphnate can be produced by the reaction of the magnesium styphnate salt with lead acetate. The presence of agents can promote the formation of specific crystalline forms.<ref>{{cite journal|author=Jacques Boileau, Claude Fauquignon, Bernard Hueber and Hans H. Meyer|title=Explosives|journal=Ullmann's Encyclopedia of Industrial Chemistry|year=2009|url=http://onlinelibrary.wiley.com/doi/10.1002/14356007.a10_143.pub2/abstract;jsessionid=E8C6E1AD587F31EFF3957D89EF442B20.f01t01|doi=10.1002/14356007.a10_143.pub2}}</ref> | ||
| ==Structure== | ==Structure== | ||
| Normal lead styphnate forms a monohydrate crystal. The α and β polymorphs are both monoclinic crystals. The lead atoms are seven coordinate and are paired via oxygen bridges. The water molecule is coordinated to the metal and is also hydrogen-bonded to the anion. Many of the Pb-O distances are short, indicating some degree of covalency. The styphnate ions lie in approximately parallel | Normal lead styphnate forms a monohydrate crystal. The α and β polymorphs are both monoclinic crystals. The lead atoms are seven coordinate and are paired via oxygen bridges. The water molecule is coordinated to the metal and is also hydrogen-bonded to the anion. Many of the Pb-O distances are short, indicating some degree of covalency. The styphnate ions lie in approximately parallel | ||
| planes linked by Pb atoms. <ref>{{cite journal|author=Pierce-Butler, M.A.|title=The structure of the lead salt of 2,4,6-trinitro-1,3-benzenediol monohydrate (alpha-polymorph)|journal=Acta. Cryst.|year=1984|url=http://scripts.iucr.org/cgi-bin/paper?S0108270184003036|doi=10.1107/S0108270184003036}}</ref><ref>{{cite journal|author=Pierce-Butler, M.A.|title=Structures of the barium salt of 2,4,6-trinitro-1,3-benzenediol monohydrate and the isomorphous lead salt (beta-polymorph)|journal=Acta. Cryst.|year=1982|url=http://scripts.iucr.org/cgi-bin/paper?S0567740882010966|doi=10.1107/S0567740882010966}}</ref> | |||
| planes linked by Pb atoms. | |||
| ==Properties== | ==Properties== | ||
| Lead styphnate has a crystal density of 3.06 to 3.1 g cm -3. It has a heat of formation of -835 kJ mol-1. The loss of water leads to the formation of a sensitive anhydride with a density of 2.9 g cm-3. Lead styphnate as a monohydrate comes in gold, orange or reddish-brown monoclinic crystals. The reason for the various colors has not been successfully explained. |
Lead styphnate has a crystal density of 3.06 to 3.1 g cm -3. It has a heat of formation of -835 kJ mol-1. The loss of water leads to the formation of a sensitive anhydride with a density of 2.9 g cm-3. Lead styphnate as a monohydrate comes in gold, orange or reddish-brown monoclinic crystals. The reason for the various colors has not been successfully explained. <ref>{{cite journal|author=Robert Matyáš, Ji í Pachman|title=Primary Explosives|journal=Springer Science & Business Media|year=2013|url=http://link.springer.com/book/10.1007%2F978-3-642-28436-6|doi=10.1007/978-3-642-28436-6}}</ref> Lead Styphnate has a detonation velocity of 5.2 km/s and an explosion temperature of 265-280°C after five seconds.<ref>{{cite journal|author=Hyman Henkin, Russell McGill |title=Rates of Explosive Decomposition of Explosives. Experimental and Theoretical Kinetic Study as a Function of Temperature|journal=Ind. Eng. Chem.|year=1952|url=http://pubs.acs.org/doi/abs/10.1021/ie50510a054|doi=10.1021/ie50510a054}}</ref> | ||
| ==Decomposition Reaction== | ==Decomposition Reaction== | ||
| :<math>C_{6}N_{3}O_{8}PbH(H_{2}O) \longrightarrow Pb(CN)_{2} + 4CO_{2} + 1/2 H_{2} + H_{2}O</math> | :<math>C_{6}N_{3}O_{8}PbH(H_{2}O) \longrightarrow Pb(CN)_{2} + 4CO_{2} + 1/2 H_{2} + H_{2}O</math> | ||
| ==Applications== | ==Applications== | ||
| Lead styphnate is mainly used in military and commercial small arm ammunition. It serves as a primary explosive because gun powder will not ignite from a simple impact. |
Lead styphnate is mainly used in military and commercial small arm ammunition. It serves as a primary explosive because gun powder will not ignite from a simple impact.<ref>{{cite journal|author=Gray, Theodore |title=Flash Bang|journal=Popular Science |year=2009|url=hhttp://www.periodictable.com/PopSci/2009/10/01/index.html}}</ref> Lead styphnate is also similarly used as primer in micro thruster for small satellite stationkeeping.<ref>{{cite journal|author=Daniel W. Youngner, et al.|title=MEMS Mega-pixel Micro-thruster Arrays for Small Satellite Stationkeeping|journal=Honeywell Technology 14th Annual/USU Conference on Small Satellites|year=2000|url=http://alfven.princeton.edu/papers/MMMAabstext.htm}}</ref> | ||
| ==References== | ==References== | ||
Revision as of 02:10, 16 December 2014
| |
| Names | |
|---|---|
| IUPAC name Lead 2,4,6-trinitrobenzene-1,3-diolate | |
Other names
| |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.035.703 |
| PubChem CID | |
| UN number | 0130 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
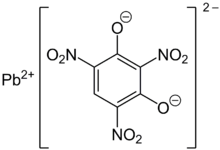
| Chemical formula | C6HN3O8Pb |
| Molar mass | 450.288 g/mol |
| Density | 3.02 g/cm, solid |
| Explosive data | |
| Shock sensitivity | High |
| Friction sensitivity | High |
| Hazards | |
| NFPA 704 (fire diamond) |
 |
| Autoignition temperature |
330 °C (626 °F; 603 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Lead styphnate (lead 2,4,6-trinitroresorcinate, C6HN3O8Pb ), whose name is derived from styphnic acid, is an explosive used as a component in primer and detonator mixtures for less sensitive secondary explosives. Lead styphnate varies in color from yellow to brown.
There are two forms of lead styphnate: six-sided monohydrate crystals and small rectangular crystals. Lead styphnate is particularly sensitive to fire and the discharge of static electricity. When dry, it can be readily detonated by static discharges from the human body. The longer and narrower the crystals, the more susceptible lead styphnate is to static electricity. Lead styphnate does not react with metals and is less sensitive to shock and friction than mercury fulminate or lead azide. Lead styphnate is only slightly soluble in water and methyl alcohol and may be neutralized by a sodium carbonate solution. It is stable in storage, even at elevated temperatures. As with other lead-containing compounds, lead styphnate is inherently toxic to humans if ingested i.e. can cause heavy metal poisoning.
Preparation
The first preparation of a lead styphnate has never been substantiated but it is thought that it could have been discovered by Peter Griess in 1874. The first well established preparation of anhydrous normal lead styphnate was by Edmund Herz in 1919, through the reaction of magnesium styphnate with lead acetate in the presence of nitric acid. Lead styphnate can be synthesized in various polymorphs and basic salts. Normal lead styphnate monohydrate, monobasic lead styphnate, tribasic lead styphnate dihydrate, and pentabasic lead styphnate dehydrate as well as α, β polymorphs of lead styphnate exist.
Lead styphnate can be produced by the reaction of the magnesium styphnate salt with lead acetate. The presence of agents can promote the formation of specific crystalline forms.
Structure
Normal lead styphnate forms a monohydrate crystal. The α and β polymorphs are both monoclinic crystals. The lead atoms are seven coordinate and are paired via oxygen bridges. The water molecule is coordinated to the metal and is also hydrogen-bonded to the anion. Many of the Pb-O distances are short, indicating some degree of covalency. The styphnate ions lie in approximately parallel planes linked by Pb atoms.
Properties
Lead styphnate has a crystal density of 3.06 to 3.1 g cm -3. It has a heat of formation of -835 kJ mol-1. The loss of water leads to the formation of a sensitive anhydride with a density of 2.9 g cm-3. Lead styphnate as a monohydrate comes in gold, orange or reddish-brown monoclinic crystals. The reason for the various colors has not been successfully explained. Lead Styphnate has a detonation velocity of 5.2 km/s and an explosion temperature of 265-280°C after five seconds.
Decomposition Reaction
Applications
Lead styphnate is mainly used in military and commercial small arm ammunition. It serves as a primary explosive because gun powder will not ignite from a simple impact. Lead styphnate is also similarly used as primer in micro thruster for small satellite stationkeeping.
References
- ECHA, European Chemicals Agency http://echa.europa.eu/documents/10162/13638/svhc_supdoc_lead_styphnate_en.pdf
- Jacques Boileau, Claude Fauquignon, Bernard Hueber and Hans H. Meyer "Explosives" in Ullmann's Encyclopedia of Industrial Chemistry 2009, Wiley-VCH, Weinheim. doi:10.1002/14356007.a10_143.pub2
- J.R. Payne (1994). "Thermochmistry of lead styphnate". Thermochimica Acta,. doi:10.1016/0040-6031(94)85003-8.
{{cite journal}}: CS1 maint: extra punctuation (link) - Jacques Boileau, Claude Fauquignon, Bernard Hueber and Hans H. Meyer (2009). "Explosives". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a10_143.pub2.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Pierce-Butler, M.A. (1984). "The structure of the lead salt of 2,4,6-trinitro-1,3-benzenediol monohydrate (alpha-polymorph)". Acta. Cryst. doi:10.1107/S0108270184003036.
- Pierce-Butler, M.A. (1982). "Structures of the barium salt of 2,4,6-trinitro-1,3-benzenediol monohydrate and the isomorphous lead salt (beta-polymorph)". Acta. Cryst. doi:10.1107/S0567740882010966.
- Robert Matyáš, Ji í Pachman (2013). "Primary Explosives". Springer Science & Business Media. doi:10.1007/978-3-642-28436-6.
- Hyman Henkin, Russell McGill (1952). "Rates of Explosive Decomposition of Explosives. Experimental and Theoretical Kinetic Study as a Function of Temperature". Ind. Eng. Chem. doi:10.1021/ie50510a054.
- Gray, Theodore (2009). . Popular Science.
- Daniel W. Youngner; et al. (2000). "MEMS Mega-pixel Micro-thruster Arrays for Small Satellite Stationkeeping". Honeywell Technology 14th Annual/USU Conference on Small Satellites.
{{cite journal}}: Explicit use of et al. in:|author=(help)

