| Revision as of 00:31, 10 February 2015 edit144.74.136.41 (talk) →External links← Previous edit | Revision as of 03:45, 18 October 2015 edit undoSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,698 edits revise to be more generalNext edit → | ||
| Line 1: | Line 1: | ||
| ] and an alpha-keto acid]] | ] and an alpha-keto acid]] | ||
| '''Transamination''' (or aminotransfer) is a chemical reaction |
'''Transamination''' (or aminotransfer) is a chemical reaction that replaces an ] ] with another amine. In many applications, an ] is the substrate. The other substrate is a ], which contains a ] (=O) group. In transamination, the NH<sub>2</sub> group on one molecule is exchanged with the =O group on the other. The original amino acid converts to a keto acid, and the keto acid converts to an amino acid. | ||
| Transamination in ] is accomplished by enzymes called ]s or aminotransferases. This process is an important step in the synthesis of some non-]s (amino acids that can be synthesized de novo by the organism). The ] of an amino acid is determined during transamination. | Transamination in ] is accomplished by enzymes called ]s or aminotransferases. This process is an important step in the synthesis of some non-]s (amino acids that can be synthesized de novo by the organism). The ] of an amino acid is determined during transamination. | ||
| Line 8: | Line 8: | ||
| ] and ] are the only two amino acids that do not always undergo transamination and rather use serine or threonine dehydrogenase. | ] and ] are the only two amino acids that do not always undergo transamination and rather use serine or threonine dehydrogenase. | ||
| A second type of transamination reaction can be described as a ] of one amine or amide anion on an amine or ammonium salt.<ref>Smith, M. B. and March, J. ''Advanced Organic Chemistry: Reactions, Mechanisms, and Structure'', 5th ed. Wiley, '''2001''', p. 503. ISBN 0-471-58589-0</ref> For example, the attack of a primary amine by a primary amide anion can be used to prepare secondary amines: | |||
| :RNH<sub>2</sub> + R'NH<sup>−</sup> → RR'NH + NH<sub>2</sub><sup>−</sup> | :RNH<sub>2</sub> + R'NH<sup>−</sup> → RR'NH + NH<sub>2</sub><sup>−</sup> | ||
| Symmetric secondary amines can be prepared using ] (2RNH<sub>2</sub> → R<sub>2</sub>NH + NH<sub>3</sub>). And finally, quaternary ammonium salts can be dealkylated using ]: | Symmetric secondary amines can be prepared using ] (2RNH<sub>2</sub> → R<sub>2</sub>NH + NH<sub>3</sub>). And finally, quaternary ammonium salts can be dealkylated using ]: | ||
| :R<sub>4</sub>N<sup>+</sup> + NH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>OH → R<sub>3</sub>N + RN<sup>+</sup>H<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>OH | :R<sub>4</sub>N<sup>+</sup> + NH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>OH → R<sub>3</sub>N + RN<sup>+</sup>H<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>OH | ||
| Aminonaphthalenes also undergo transaminations.<ref name=Ullmann>Gerald Booth "Naphthalene Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. {{DOI|10.1002/14356007.a17_009}}.</ref> | |||
| ==References== | ==References== | ||
Revision as of 03:45, 18 October 2015
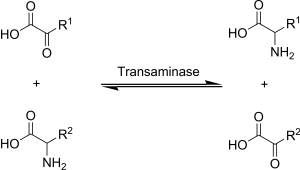
Transamination (or aminotransfer) is a chemical reaction that replaces an amine functional group with another amine. In many applications, an amino acid is the substrate. The other substrate is a keto acid, which contains a keto (=O) group. In transamination, the NH2 group on one molecule is exchanged with the =O group on the other. The original amino acid converts to a keto acid, and the keto acid converts to an amino acid.
Transamination in biochemistry is accomplished by enzymes called transaminases or aminotransferases. This process is an important step in the synthesis of some non-essential amino acids (amino acids that can be synthesized de novo by the organism). The chirality of an amino acid is determined during transamination. This reaction uses the coenzyme PLP, and has been shown to be a kinetically perfect reaction. The product of transamination reactions depend on the availability of alpha-keto acids. The products usually are either alanine, aspartate or glutamate, since their corresponding alpha-keto acids are produced through metabolism of fuels.
Serine and threonine are the only two amino acids that do not always undergo transamination and rather use serine or threonine dehydrogenase.
A second type of transamination reaction can be described as a nucleophilic substitution of one amine or amide anion on an amine or ammonium salt. For example, the attack of a primary amine by a primary amide anion can be used to prepare secondary amines:
- RNH2 + R'NH → RR'NH + NH2
Symmetric secondary amines can be prepared using Raney nickel (2RNH2 → R2NH + NH3). And finally, quaternary ammonium salts can be dealkylated using ethanolamine:
- R4N + NH2CH2CH2OH → R3N + RNH2CH2CH2OH
Aminonaphthalenes also undergo transaminations.
References
- Smith, M. B. and March, J. Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 5th ed. Wiley, 2001, p. 503. ISBN 0-471-58589-0
- Gerald Booth "Naphthalene Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a17_009.
External links
- Overview of amino acid synthesis (dead link)
- The chemical logic behind aminoacid degradation and the urea cycle (dead link)