| Revision as of 22:59, 3 June 2016 view sourcePlantsurfer (talk | contribs)Extended confirmed users40,052 edits →Copper Age: invalid template field in ref← Previous edit | Revision as of 23:12, 3 June 2016 view source Sfarney (talk | contribs)Extended confirmed users3,974 edits →Chemical: Copyedit; add historic shift to aluminum wiring.Tags: nowiki added Visual editNext edit → | ||
| Line 33: | Line 33: | ||
| ] | ] | ||
| ]. The contrast between the refurbished copper installed in 2010 and the green color of the original 1894 copper is clearly seen.]] | ]. The contrast between the refurbished copper installed in 2010 and the green color of the original 1894 copper is clearly seen.]] | ||
| Copper does not react with water but it does slowly react with atmospheric oxygen to form a layer of brown-black copper oxide which, unlike the ] that forms on iron in moist air, protects the underlying metal from further corrosion (]). A green layer of ] (copper carbonate) can often be seen on old copper structures, such as the roofing of many older buildings<ref>{{Cite book|url=https://books.google.com/books?id=3qL3vfUZHMYC|title=Cultural Heritage Conservation and Environmental Impact Assessment by Non-Destructive Testing and Micro-Analysis|last=Grieken|first=Rene van|last2=Janssens|first2=Koen|date=2005-09-27|publisher=CRC Press|isbn=9780203970782|page=197|language=en}}</ref> and the ].<ref>{{cite web|title=Copper.org: Education: Statue of Liberty: Reclothing the First Lady of Metals – Repair Concerns|url=http://www.copper.org/education/liberty/liberty_reclothed1.html|work=Copper.org|accessdate=11 April 2011}}</ref> Copper ]es when exposed to ], with which it reacts to form various ]s.<ref>{{cite journal|last1=Rickett|first1=B. I.|last2=Payer|first2=J. H.|title=Composition of Copper Tarnish Products Formed in Moist Air with Trace Levels of Pollutant Gas: Hydrogen Sulfide and Sulfur Dioxide/Hydrogen Sulfide|journal=Journal of the Electrochemical Society|date=1995|volume=142|issue=11|pages=3723–3728|doi=10.1149/1.2048404}}</ref> | Copper does not react with water but it does slowly react with atmospheric oxygen to form a layer of brown-black copper oxide which, unlike the ] that forms on iron in moist air, protects the underlying metal from further corrosion (]). A green layer of ] (copper carbonate) can often be seen on old copper structures, such as the roofing of many older buildings<ref name=":0">{{Cite book|url=https://books.google.com/books?id=3qL3vfUZHMYC|title=Cultural Heritage Conservation and Environmental Impact Assessment by Non-Destructive Testing and Micro-Analysis|last=Grieken|first=Rene van|last2=Janssens|first2=Koen|date=2005-09-27|publisher=CRC Press|isbn=9780203970782|page=197|language=en}}</ref> and the ].<ref>{{cite web|title=Copper.org: Education: Statue of Liberty: Reclothing the First Lady of Metals – Repair Concerns|url=http://www.copper.org/education/liberty/liberty_reclothed1.html|work=Copper.org|accessdate=11 April 2011}}</ref> Copper ]es when exposed to some ] compounds, with which it reacts to form various ]s.<ref>{{cite journal|last1=Rickett|first1=B. I.|last2=Payer|first2=J. H.|title=Composition of Copper Tarnish Products Formed in Moist Air with Trace Levels of Pollutant Gas: Hydrogen Sulfide and Sulfur Dioxide/Hydrogen Sulfide|journal=Journal of the Electrochemical Society|date=1995|volume=142|issue=11|pages=3723–3728|doi=10.1149/1.2048404}}</ref> | ||
| ===Isotopes=== | ===Isotopes=== | ||
| Line 53: | Line 53: | ||
| {{see also|List of countries by copper production}} | {{see also|List of countries by copper production}} | ||
| Most copper is mined or ] as copper sulfides from large ]s in ] deposits that contain 0.4 to 1.0% copper. Sites include ] in Chile, ] in Utah, United States and ] in New Mexico, United States. According to the ] |
Most copper is mined or ] as copper sulfides from large ]s in ] deposits that contain 0.4 to 1.0% copper. Sites include ] in Chile, ] in Utah, United States and ] in New Mexico, United States. According to the ] in 2005, Chile was the top producer of copper with at least one-third world share followed by the United States, Indonesia and Peru.<ref name=CRC/> Copper can also be recovered through the ] process. Several sites in the state of Arizona are considered prime candidates for this method.<ref>{{cite web|last=Randazzo |first=Ryan |url=http://www.azcentral.com/arizonarepublic/business/articles/2011/06/19/20110619copper-new-method-fight.html |title=A new method to harvest copper |publisher=Azcentral.com |date=19 June 2011 |accessdate=25 April 2014}}</ref> The amount of copper in use is increasing and the quantity available is barely sufficient to allow all countries to reach developed world levels of usage.<ref>{{cite journal|url=http://www.pnas.org/content/103/5/1209.full|title=Metal stocks and sustainability|journal=PNAS|date=2006|volume=103|issue=5|pages=1209–1214|first1=R. B.|last1=Gordon|first2=M.|last2=Bertram|first3=T. E.|last3=Graedel|doi=10.1073/pnas.0509498103|pmc=1360560|pmid=16432205|bibcode = 2006PNAS..103.1209G}}</ref> | ||
| ===Reserves=== | ===Reserves=== | ||
| Line 135: | Line 135: | ||
| ], ], Israel.]] | ], ], Israel.]] | ||
| In Greece, copper was known by the name ''chalkos'' (χαλκός). It was an important resource for the Romans, Greeks and other ancient peoples. In Roman times, it was known as ''aes Cyprium'', ''aes'' being the generic Latin term for copper alloys and ''Cyprium'' from ], where much copper was mined. The phrase was simplified to ''cuprum'', hence the English ''copper''. ] |
In Greece, copper was known by the name ''chalkos'' (χαλκός). It was an important resource for the Romans, Greeks and other ancient peoples. In Roman times, it was known as ''aes Cyprium'', ''aes'' being the generic Latin term for copper alloys and ''Cyprium'' from ], where much copper was mined. The phrase was simplified to ''cuprum'', hence the English ''copper''. ] (]<nowiki/>rin Rome)epresented copper in mythology and alchemy because of its lustrous beauty, its ancient use in producing mirrors; Cyprus was sacred to the goddess. The seven heavenly bodies known to the ancients were associated with the seven metals known in antiquity, and Venus was assigned to copper.<ref>{{cite journal|title = The Nomenclature of Copper and its Alloys|author = Rickard, T. A. |journal = Journal of the Royal Anthropological Institute|volume = 62|date = 1932|page=281|jstor = 2843960|doi = 10.2307/2843960|publisher = Royal Anthropological Institute}}</ref> | ||
| Britain's first |
Britain's first used brass in about the 3rd or 2nd Century BC. In North America, copper mining began with marginal workings by Native Americans. Native copper is known to have been extracted from sites on ] with primitive stone tools between 800 and 1600.<ref>{{cite journal|title = The State of Our Knowledge About Ancient Copper Mining in Michigan|journal = The Michigan Archaeologist|volume = 41|page = 119|author = Martin, Susan R. |date = 1995|url = http://www.ramtops.co.uk/copper.html|issue =2–3}}</ref> Copper metallurgy was flourishing in South America, particularly in Peru around 1000 AD; it proceeded at a much slower rate on other continents.{{Clarification needed|reason=Since South America was one of the last to develop copper, what "other continents" were slower?|date=June 2016}} Copper burial ornamentals from the 15th century have been uncovered, but the metal's commercial production did not start until the early 20th century. | ||
| The cultural role of copper has been important, particularly in currency. Romans in the 6th through 3rd centuries BC used copper lumps as money. At first, the copper itself was valued, but gradually the shape and look of the copper became more important. ] had his own coins made from brass, while ]'s coins were made from Cu-Pb-Sn alloys. With an estimated annual output of around 15,000 t, ] reached a scale unsurpassed until the time of the ]; the ] most intensely mined were those of ], ] and in Central Europe.<ref>{{cite journal|doi = 10.1126/science.272.5259.246|title = History of Ancient Copper Smelting Pollution During Roman and Medieval Times Recorded in Greenland Ice|pages = 246–249 (247f.)|date = 1996|last1 = Hong|first1 = S.|last2 = Candelone|first2 = J.-P.|issue = 5259|last3 = Patterson|first3 = C. C.|last4 = Boutron|first4 = C. F.|journal = Science|volume = 272|bibcode = 1996Sci...272..246H}}</ref><ref>{{cite journal|last = de Callataÿ|first = François|date = 2005|title = The Graeco-Roman Economy in the Super Long-Run: Lead, Copper, and Shipwrecks|journal = Journal of Roman Archaeology|volume = 18|pages = 361–372 (366–369)}}</ref> | The cultural role of copper has been important, particularly in currency. Romans in the 6th through 3rd centuries BC used copper lumps as money. At first, the copper itself was valued, but gradually the shape and look of the copper became more important. ] had his own coins made from brass, while ]'s coins were made from Cu-Pb-Sn alloys. With an estimated annual output of around 15,000 t, ] reached a scale unsurpassed until the time of the ]; the ] most intensely mined were those of ], ] and in Central Europe.<ref>{{cite journal|doi = 10.1126/science.272.5259.246|title = History of Ancient Copper Smelting Pollution During Roman and Medieval Times Recorded in Greenland Ice|pages = 246–249 (247f.)|date = 1996|last1 = Hong|first1 = S.|last2 = Candelone|first2 = J.-P.|issue = 5259|last3 = Patterson|first3 = C. C.|last4 = Boutron|first4 = C. F.|journal = Science|volume = 272|bibcode = 1996Sci...272..246H}}</ref><ref>{{cite journal|last = de Callataÿ|first = François|date = 2005|title = The Graeco-Roman Economy in the Super Long-Run: Lead, Copper, and Shipwrecks|journal = Journal of Roman Archaeology|volume = 18|pages = 361–372 (366–369)}}</ref> | ||
| The gates of the ] used ] |
The gates of the ] used ] treated with ].{{Clarification needed|reason=Bronze is not a gold alloy, but depletion gilding can be done only on gold alloy.|date=June 2016}} The process was most prevalent in ], where alchemy is thought to have begun.<ref>{{cite journal|url = http://www.goldbulletin.org/downloads/JACOB_2_33.PDF|title = Corinthian Bronze and the Gold of the Alchemists|author=Savenije, Tom J. |author2=Warman, John M. |author3=Barentsen, Helma M. |author4=van Dijk, Marinus |author5=Zuilhof, Han |author6=Sudhölter, Ernst J. R.|journal = Macromolecules|issue = 2|volume = 33|date = 2000|page=60|doi = 10.1021/ma9904870|bibcode = 2000MaMol..33...60S}}</ref>{{Dead link|date=June 2016}} In ancient ], copper was used in the ] medical science ] for ] instruments and other medical equipment. Ancient ] (~2400 BC) used copper for sterilizing wounds and drinking water, and later to treat headaches, burns, and itching. The ], with copper cylinders soldered to lead, dates back to 248 BC to AD 226{{Clarification needed|reason=This is a huge range of dates with apparently precise endpoints. What does it mean? The text has no meaning as currently worded.|date=June 2016}} and resembles a galvanic cell, leading some people to believe this was the first battery; the hypothesis has not been verified.<ref>{{cite web|url=http://www.world-mysteries.com/strange-artifacts/out-of-place-artifacts/the-baghdad-battery/|title=World Mysteries – Strange Artifacts, Baghdad Battery|work=World-Mysteries.com|accessdate=22 April 2011}}</ref> | ||
| ===Modern period=== | ===Modern period=== | ||
| ] affecting the stream running from the disused ] copper mines]] | ] affecting the stream running from the disused ] copper mines]] | ||
| The ] was a mine in Falun, Sweden, that operated from the 10th century to 1992. It |
The ] was a mine in Falun, Sweden, that operated from the 10th century to 1992. It satisfied two thirds of Europe's copper consumption in the 17th century and helped fund many of Sweden's wars during that time.<ref>{{cite book|url = https://books.google.com/books?id=4yp-x3TzDnEC&pg=PA60|page = 60|title = Mining in World History|isbn = 978-1-86189-173-0|author1 = Lynch, Martin|date = 15 April 2004}}</ref> It was referred to as the nation's treasury; Sweden had a ].<ref>{{cite web|title=Gold: prices, facts, figures and research: A brief history of money|url=http://www.galmarley.com/FAQs_pages/monetary_history_faqs.htm#Scandinavian%20copper%20money|accessdate=22 April 2011}}</ref> | ||
| Copper was used in roofing,<ref name=":0" /> currency, ] sculpture, photographic technology known as the ], the ], and other structures. ] and ] was widely used in the hulls of ships, of which the ships of Christopher Columbus were among the earliest.<ref>{{cite web|title = Copper History|url = http://www.copperinfo.com/aboutcopper/history.html|accessdate = 4 September 2008}}</ref> The ] in Hamburg was the first modern ] plant starting its production in 1876.<ref>{{cite journal|doi = 10.1002/adem.200400403|title = Process Optimization in Copper Electrorefining|date = 2004|author = Stelter, M.|journal = Advanced Engineering Materials|volume = 6|issue = 7|page=558|last2 = Bombach|first2 = H.}}</ref> The German scientist ] invented ] in 1830 while determining the metal's atomic mass; around then it was discovered that the amount and type of alloying element (e.g., tin) to copper would affect bell tones. ] was developed by ] in Finland and first applied at ] in 1949; the energy-efficient process accounts for 50% of the world's primary copper production.<ref>{{cite web|url = http://www.outokumpu.com/files/Technology/Documents/Newlogobrochures/FlashSmelting.pdf|archiveurl = https://web.archive.org/web/20110724043222/http://www.outokumpu.com/files/Technology/Documents/Newlogobrochures/FlashSmelting.pdf|archivedate = 24 July 2011|title = Outokumpu Flash Smelting|publisher = ]|page = 2}}{{dead link|date=July 2012}}</ref> | |||
| The ], formed in 1967 |
The ], formed in 1967 by Chile, Peru, Zaire and Zambia, operates in the copper market as ] does for oil, though it never achieved the same influence, particularly because the second-largest producer, the United States, was never a member; it was dissolved in 1988.<ref>{{cite journal |author=Karen A. Mingst |date=1976 |title=Cooperation or illusion: an examination of the intergovernmental council of copper exporting countries |journal=International Organization |volume=30 |issue=2 |pages=263–287 |doi=10.1017/S0020818300018270}}</ref> | ||
| ==Applications== | ==Applications== | ||
| {{See also| Copper in renewable energy}} | {{See also| Copper in renewable energy}} | ||
| ] | ] | ||
| The major applications of copper are in electrical wires (60%), roofing and plumbing (20%) and industrial machinery (15%). Copper is mostly used as a pure metal, but when a higher hardness is required it is |
The major applications of copper are in electrical wires (60%), roofing and plumbing (20%), and industrial machinery (15%). Copper is mostly used as a pure metal, but when a higher hardness is required, it is alloyed with other elements (5% of total use) such as ] and ].<ref name=emsley/> For more than two centuries, copper paint has been used on boat hulls to control the growth of plants and shellfish.<ref>{{Cite web|url=http://www.boatus.com/magazine/2012/february/copper.asp|title=Is Copper Bottom Paint Sinking? - BoatUS Magazine|website=http://www.boatus.com|access-date=2016-06-03}}</ref> A small part of the copper supply is used for nutritional supplements and fungicides in agriculture.<ref name="Boux"/><ref name="Applications for Copper">{{cite web|title = Copper|publisher = ]|date = 2008|url = http://www.americanelements.com/cu.html|accessdate = 12 July 2008}}</ref> ] of copper is possible, although alloys are preferred for good ] characteristics in creating intricate parts. | ||
| ===Wire and cable=== | ===Wire and cable=== | ||
| {{Main| Copper wire and cable}} | {{Main| Copper wire and cable}} | ||
| Despite competition from other materials, copper remains the preferred ] in nearly all categories of electrical wiring with the major exception being overhead ] where ] is often preferred.<ref>Pops, Horace, 2008, Processing of wire from antiquity to the future, Wire Journal International, June, pp 58–66</ref><ref>The Metallurgy of Copper Wire, http://www.litz-wire.com/pdf%20files/Metallurgy_Copper_Wire.pdf</ref> Copper wire is used in ], ], ], ], ] circuitry, and countless types of ].<ref>Joseph, Günter, 1999, Copper: Its Trade, Manufacture, Use, and Environmental Status, edited by Kundig, Konrad J.A., ASM International, pps. 141–192 and pps. 331–375.</ref> ] is the most important market for the copper industry.<ref>{{cite web|url=http://www.chemistryexplained.com/elements/C-K/Copper.html |title=Copper, Chemical Element – Overview, Discovery and naming, Physical properties, Chemical properties, Occurrence in nature, Isotopes |publisher=Chemistryexplained.com |accessdate=16 October 2012}}</ref> This includes |
Despite competition from other materials, copper remains the preferred ] in nearly all categories of electrical wiring with the major exception being overhead ] where ] is often preferred.<ref>Pops, Horace, 2008, Processing of wire from antiquity to the future, Wire Journal International, June, pp 58–66</ref><ref>The Metallurgy of Copper Wire, http://www.litz-wire.com/pdf%20files/Metallurgy_Copper_Wire.pdf</ref> Copper wire is used in ], ], ], ], ] circuitry, and countless types of ].<ref>Joseph, Günter, 1999, Copper: Its Trade, Manufacture, Use, and Environmental Status, edited by Kundig, Konrad J.A., ASM International, pps. 141–192 and pps. 331–375.</ref> ] is the most important market for the copper industry.<ref>{{cite web|url=http://www.chemistryexplained.com/elements/C-K/Copper.html |title=Copper, Chemical Element – Overview, Discovery and naming, Physical properties, Chemical properties, Occurrence in nature, Isotopes |publisher=Chemistryexplained.com |accessdate=16 October 2012}}</ref> This includes structural power wiring, power distribution cable, appliance wire, communications cable, automotive wire and cable, and magnet wire. Roughly half of all copper mined is used to manufacture electrical wire and cable conductors.<ref>Joseph, Günter, 1999, Copper: Its Trade, Manufacture, Use, and Environmental Status, edited by Kundig, Konrad J.A., ASM International, p.348</ref> Many electrical devices rely on copper wiring because of its multitude of inherent beneficial properties, such as its high ], ], ], ] resistance, ] resistance, low ], high ], ]ability, and ease of installation. | ||
| For a short period from the late 1960s to the late 1970s, copper wiring was replaced by ] in many housing construction projects in America ''(see ] for main article)''. The new wiring was implicated in a number of house fires and the industry returned to copper.<ref>{{Cite web|url=http://www.heimer.com/information/aluminum_wiring.html|title=The Requested Page has moved.|website=www.heimer.com|access-date=2016-06-03}}</ref><ref>{{Cite web|url=http://www.faqs.org/faqs/electrical-wiring/part2/section-16.html|title=Electrical Wiring FAQ (Part 2 of 2)Section - Aluminum wiring|website=www.faqs.org|access-date=2016-06-03}}</ref> | |||
| ===Electronics and related devices=== | ===Electronics and related devices=== | ||
| Line 168: | Line 170: | ||
| {{Main|Copper in energy efficient motors}} | {{Main|Copper in energy efficient motors}} | ||
| Copper's |
Copper's superior ] enhances the efficiency of electrical ].<ref>IE3 energy-saving motors, Engineer Live, http://www.engineerlive.com/Design-Engineer/Motors_and_Drives/IE3_energy-saving_motors/22687/</ref> This is important because motors and motor-driven systems account for 43%-46% of all global electricity consumption and 69% of all electricity used by industry.<ref>Energy‐efficiency policy opportunities for electric motor‐driven systems, International Energy Agency, 2011 Working Paper in the Energy Efficiency Series, by Paul Waide and Conrad U. Brunner, OECD/IEA 2011</ref> Increasing the mass and cross section of copper in a ] increases the electrical energy efficiency of the motor. ], a new technology designed for motor applications where energy savings are prime design objectives,<ref>Fuchsloch, J. and E.F. Brush, (2007), "Systematic Design Approach for a New Series of Ultra‐NEMA Premium Copper Rotor Motors", in EEMODS 2007 Conference Proceedings, 10‐15 June,Beijing.</ref><ref>Copper motor rotor project; Copper Development Association; http://www.copper.org/applications/electrical/motor-rotor</ref> are enabling general-purpose ]s to meet and exceed ] (NEMA) ] standards.<ref>NEMA Premium Motors, The Association of Electrical Equipment and Medical Imaging Manufacturers; http://www.nema.org/gov/energy/efficiency/premium/</ref> | ||
| ===Architecture=== | ===Architecture=== | ||
Revision as of 23:12, 3 June 2016
For other uses, see Copper (disambiguation).
Chemical element with atomic number 29 (Cu)
Copper is a chemical element with symbol Cu (from Template:Lang-la) and atomic number 29. It is a soft, malleable and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a reddish-orange color. It is used as a conductor of heat and electricity, as a building material and as a constituent of various metal alloys, such as Sterling silver used in jewelry, cupronickel used to make marine hardware and coins and constantan used in strain gauges and thermocouples for temperature measurement.
Copper is found as a pure metal in nature, and this was the first source of the metal to be used by humans, ca. 8,000 BC. It was the first metal to be smelted from its ore, ca. 5,000 BC, the first metal to be cast into a shape in a mold, ca. 4,000 BC and the first metal to be purposefully alloyed with another metal, tin, to create bronze, ca. 3,500 BC.
In the Roman era, copper was principally mined on Cyprus, the origin of the name of the metal, from aes сyprium (metal of Cyprus), later corrupted to сuprum, from which the words copper (English), cuivre (French), Koper (Dutch) and Kupfer (German) are all derived. The commonly encountered compounds are copper(II) salts, which often impart blue or green colors to such minerals as azurite, malachite, and turquoise, and have been used widely and historically as pigments. Architectural structures built with copper (usually roofing elements) corrode to give green verdigris (or patina). Decorative art prominently features copper, both in the elemental metal and in compounds as pigments.
Copper is essential to all living organisms as a trace dietary mineral because it is a key constituent of the respiratory enzyme complex cytochrome c oxidase. In molluscs and crustacea copper is a constituent of the blood pigment hemocyanin, replaced by the iron-complexed hemoglobin in fish and other vertebrates. In humans, copper is found mainly in the liver, muscle, and bone. The adult body contains between 1.4 and 2.1 mg of copper per kilogram of body weight. Hence a healthy human weighing 60 kilogram contains approximately 0.1g of copper. However, this small amount is essential to the overall human well-being.
Commercially, copper compounds are used as bacteriostatic agents, fungicides, and wood preservatives.
Characteristics
Physical


Copper, silver and gold are in group 11 of the periodic table, and they share certain attributes: they have one s-orbital electron on top of a filled d-electron shell and are characterized by high ductility and electrical conductivity. The filled d-shells in these elements contribute little to interatomic interactions, which are dominated by the s-electrons through metallic bonds. Unlike metals with incomplete d-shells, metallic bonds in copper are lacking a covalent character and are relatively weak. This observation explains the low hardness and high ductility of single crystals of copper. At the macroscopic scale, introduction of extended defects to the crystal lattice, such as grain boundaries, hinders flow of the material under applied stress, thereby increasing its hardness. For this reason, copper is usually supplied in a fine-grained polycrystalline form, which has greater strength than monocrystalline forms.
The softness of copper partly explains its high electrical conductivity (59.6×10 S/m) and high thermal conductivity, the second highest (second only to silver) among pure metals at room temperature. This is because the resistivity to electron transport in metals at room temperature originates primarily from scattering of electrons on thermal vibrations of the lattice, which are relatively weak in a soft metal. The maximum permissible current density of copper in open air is approximately 3.1×10 A/m of cross-sectional area, above which it begins to heat excessively.
Copper is one of four metallic elements with a natural color other than gray or silver, the others being caesium (yellow), gold (yellow), and osmium (bluish). Pure copper is orange-red and acquires a reddish tarnish when exposed to air. The characteristic color of copper results from the electronic transitions between the filled 3d and half-empty 4s atomic shells – the energy difference between these shells corresponds to orange light. The same mechanism causes the yellow color of gold and caesium.
As with other metals, if copper is put in contact with another metal, galvanic corrosion will occur.
Chemical


Copper does not react with water but it does slowly react with atmospheric oxygen to form a layer of brown-black copper oxide which, unlike the rust that forms on iron in moist air, protects the underlying metal from further corrosion (passivation). A green layer of verdigris (copper carbonate) can often be seen on old copper structures, such as the roofing of many older buildings and the Statue of Liberty. Copper tarnishes when exposed to some sulfur compounds, with which it reacts to form various copper sulfides.
Isotopes
Main article: Isotopes of copperThere are 29 isotopes of copper. Cu and Cu are stable, with Cu comprising approximately 69% of naturally occurring copper; both have a spin of 3⁄2. The other isotopes are radioactive, with the most stable being Cu with a half-life of 61.83 hours. Seven metastable isotopes have been characterized; Cu is the longest-lived with a half-life of 3.8 minutes. Isotopes with a mass number above 64 decay by β, whereas those with a mass number below 64 decay by β. Cu, which has a half-life of 12.7 hours, decays both ways.
Cu and Cu have significant applications. Cu is used in Cu-PTSM as a radioactive tracer for positron emission tomography.
Occurrence

Copper is produced in massive stars and is present in the Earth's crust in a proportion of about 50 parts per million (ppm). It occurs as native copper, in the copper sulfides chalcopyrite and chalcocite, in the copper carbonates azurite and malachite, and in the copper(I) oxide mineral cuprite. The largest mass of elemental copper discovered weighed 420 tonnes and was found in 1857 on the Keweenaw Peninsula in Michigan, US. Native copper is a polycrystal, with the largest single crystal ever described measuring 4.4×3.2×3.2 cm.
Production

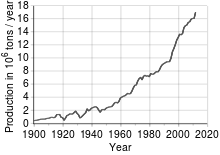

Most copper is mined or extracted as copper sulfides from large open pit mines in porphyry copper deposits that contain 0.4 to 1.0% copper. Sites include Chuquicamata in Chile, Bingham Canyon Mine in Utah, United States and El Chino Mine in New Mexico, United States. According to the British Geological Survey in 2005, Chile was the top producer of copper with at least one-third world share followed by the United States, Indonesia and Peru. Copper can also be recovered through the in-situ leach process. Several sites in the state of Arizona are considered prime candidates for this method. The amount of copper in use is increasing and the quantity available is barely sufficient to allow all countries to reach developed world levels of usage.
Reserves
See also: Peak copper § ReservesCopper has been in use at least 10,000 years, but more than 95% of all copper ever mined and smelted has been extracted since 1900, and more than half was extracted the last 24 years. As with many natural resources, the total amount of copper on Earth is vast, with around 10 tons in the top kilometer of Earth's crust, which is about 5 million years' worth at the current rate of extraction. However, only a tiny fraction of these reserves is economically viable with present-day prices and technologies. Estimates of copper reserves available for mining vary from 25 years to 60 years, depending on core assumptions such as the growth rate. Recycling is a major source of copper in the modern world. Because of these and other factors, the future of copper production and supply is the subject of much debate, including the concept of peak copper, analogous to peak oil.
The price of copper has historically been unstable, and it sextupled from the 60-year low of US$0.60/lb (US$1.32/kg) in June 1999 to US$3.75 per pound (US$8.27/kg) in May 2006. It dropped to US$2.40/lb (US$5.29/kg) in February 2007, then rebounded to US$3.50/lb (US$7.71/kg) in April 2007. In February 2009, weakening global demand and a steep fall in commodity prices since the previous year's highs left copper prices at US$1.51/lb (US$3.32/kg).
Methods
Main article: Copper extraction techniques
The concentration of copper in ores averages only 0.6%, and most commercial ores are sulfides, especially chalcopyrite (CuFeS2) and to a lesser extent chalcocite (Cu2S). These minerals are concentrated from crushed ores to the level of 10–15% copper by froth flotation or bioleaching. Heating this material with silica in flash smelting removes much of the iron as slag. The process exploits the greater ease of converting iron sulfides into oxides, which in turn react with the silica to form the silicate slag that floats on top of the heated mass. The resulting copper matte, consisting of Cu2S, is roasted to convert all sulfides into oxides:
- 2 Cu2S + 3 O2 → 2 Cu2O + 2 SO2
The cuprous oxide is converted to blister copper upon heating:
- 2 Cu2O → 4 Cu + O2
The Sudbury matte process converted only half the sulfide to oxide and then used this oxide to remove the rest of the sulfur as oxide. It was then electrolytically refined and the anode mud exploited for the platinum and gold it contained. This step exploits the relatively easy reduction of copper oxides to copper metal. Natural gas is blown across the blister to remove most of the remaining oxygen and electrorefining is performed on the resulting material to produce pure copper:
- Cu + 2 e → Cu
Recycling
Like aluminium, copper is 100% recyclable without any loss of quality, both from raw state and from manufactured products. In volume, copper is the third most recycled metal after iron and aluminium. An estimated 80% of all copper ever mined is still in use today. According to the International Resource Panel's Metal Stocks in Society report, the global per capita stock of copper in use in society is 35–55 kg. Much of this is in more-developed countries (140–300 kg per capita) rather than less-developed countries (30–40 kg per capita).
The process of recycling copper is roughly the same as is used to extract copper but requires fewer steps. High-purity scrap copper is melted in a furnace and then reduced and cast into billets and ingots; lower-purity scrap is refined by electroplating in a bath of sulfuric acid.
Alloys
See also: List of copper alloysNumerous copper alloys have been formulated, many with important uses. Brass is an alloy of copper and zinc. Bronze usually refers to copper-tin alloys, but can refer to any alloy of copper such as aluminium bronze. Copper is one of the most important constituents of carat silver and gold alloys, and carat solders are used in the jewelry industry, modifying the color, hardness and melting point of the resulting alloys.
The alloy of copper and nickel, called cupronickel, is used in low-denomination coins, often for the outer cladding. The US 5-cent coin (currently called a nickel) consists of 75% copper and 25% nickel in homogeneous composition. The alloy of 90% copper and 10% nickel, remarkable for its resistance to corrosion, is used for various objects exposed to seawater, though it is vulnerable to the sulfides sometimes found in polluted harbors and estuaries. Alloys of copper with aluminium (about 7%) have a pleasant golden color and are used in decorations. Some lead-free solders consist of tin alloyed with a small proportion of copper and other metals.
Compounds

Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively.
Binary compounds
As with other elements, the simplest compounds of copper are binary compounds, i.e. those containing only two elements, the principal examples being oxides, sulfides, and halides. Both cuprous and cupric oxides are known. Among the numerous copper sulfides, important examples include copper(I) sulfide and copper(II) sulfide.
Cuprous halides (with chlorine, bromine, and iodine) are known, as are cupric halides with fluorine, chlorine, and bromine. Attempts to prepare copper(II) iodide yield only cuprous iodide and iodine.
- 2 Cu + 4 I → 2 CuI + I2
Coordination chemistry

Copper, like all metals, forms coordination complexes with ligands. In aqueous solution, copper(II) exists as . This complex exhibits the fastest water exchange rate (speed of water ligands attaching and detaching) for any transition metal aquo complex. Adding aqueous sodium hydroxide causes the precipitation of light blue solid copper(II) hydroxide. A simplified equation is:
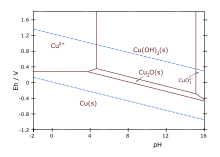
- Cu + 2 OH → Cu(OH)2
Aqueous ammonia results in the same precipitate. Upon adding excess ammonia, the precipitate dissolves, forming tetraamminecopper(II):
- Cu(H2O)4(OH)2 + 4 NH3 → + 2 H2O + 2 OH
Many other oxyanions form complexes; these include copper(II) acetate, copper(II) nitrate, and copper(II) carbonate. Copper(II) sulfate forms a blue crystalline pentahydrate, the most familiar copper compound in the laboratory. It is used in a fungicide called the Bordeaux mixture.

Polyols, compounds containing more than one alcohol functional group, generally interact with cupric salts. For example, copper salts are used to test for reducing sugars. Specifically, using Benedict's reagent and Fehling's solution the presence of the sugar is signaled by a color change from blue Cu(II) to reddish copper(I) oxide. Schweizer's reagent and related complexes with ethylenediamine and other amines dissolve cellulose. Amino acids form very stable chelate complexes with copper(II). Many wet-chemical tests for copper ions exist, one involving potassium ferrocyanide, which gives a brown precipitate with copper(II) salts.
Organocopper chemistry
Main article: Organocopper compoundCompounds that contain a carbon-copper bond are known as organocopper compounds. They are very reactive towards oxygen to form copper(I) oxide and have many uses in chemistry. They are synthesized by treating copper(I) compounds with Grignard reagents, terminal alkynes or organolithium reagents; in particular, the last reaction described produces a Gilman reagent. These can undergo substitution with alkyl halides to form coupling products; as such, they are important in the field of organic synthesis. Copper(I) acetylide is highly shock-sensitive but is an intermediate in reactions such as the Cadiot-Chodkiewicz coupling and the Sonogashira coupling. Conjugate addition to enones and carbocupration of alkynes can also be achieved with organocopper compounds. Copper(I) forms a variety of weak complexes with alkenes and carbon monoxide, especially in the presence of amine ligands.
Copper(III) and copper(IV)
Copper(III) is most often found in oxides. A simple example is potassium cuprate, KCuO2, a blue-black solid. The most extensively studied copper(III) compounds are the cuprate superconductors. Yttrium barium copper oxide (YBa2Cu3O7) consists of both Cu(II) and Cu(III) centres. Like oxide, fluoride is a highly basic anion and is known to stabilize metal ions in high oxidation states. Both copper(III) and even copper(IV) fluorides are known, K3CuF6 and Cs2CuF6, respectively.
Some copper proteins form oxo complexes, which also feature copper(III). With tetrapeptides, purple-colored copper(III) complexes are stabilized by the deprotonated amide ligands.
Complexes of copper(III) are also found as intermediates in reactions of organocopper compounds.
History
Copper Age
Main article: Copper Age


Copper occurs naturally as native metallic copper and was known to some of the oldest civilizations on record. The history of copper use is at least 11,000 years old, estimated to have begun in 9000 BC in the Middle East; a copper pendant was found in northern Iraq that dates to 8700 BC. Evidence suggests that gold and meteoric iron (but not iron smelting) were the only metals used by humans before copper. The history of copper metallurgy is thought to follow this sequence: 1) cold working of native copper, 2) annealing, 3) smelting, and 4) the lost wax casting. In southeastern Anatolia, all four of these techniques appear more or less simultaneously at the beginning of the Neolithic c. 7500 BC.
Just as agriculture was independently invented in several parts of the world, copper smelting was independently invented in different places. It was probably discovered in China before 2800 BC, in Central America perhaps around 600 AD, and in West Africa about the 9th or 10th century AD. Investment casting was invented in 4500–4000 BC in Southeast Asia and carbon dating has established mining at Alderley Edge in Cheshire, UK at 2280 to 1890 BC. Ötzi the Iceman, a male dated from 3300–3200 BC, was found with an axe with a copper head 99.7% pure; high levels of arsenic in his hair suggest his involvement in copper smelting. Experience with copper has assisted the development of other metals; in particular, copper smelting led to the discovery of iron smelting. Production in the Old Copper Complex in Michigan and Wisconsin is dated between 6000 and 3000 BC. Natural bronze, a type of copper made from ores rich in silicon, arsenic, and (rarely) tin, came into general use in the Balkans around 5500 BC.
Bronze Age
Main article: Bronze AgeAlloying copper with tin to make bronze was first practiced about 4000 years after the discovery of copper smelting, and about 2000 years after "natural bronze" had come into general use. Bronze artifacts from the Vinča culture date to 4500 BC. Sumerian and Egyptian artifacts of copper and bronze alloys date to 3000 BC. The Bronze Age began in Southeastern Europe around 3700–3300 BC, in Northwestern Europe about 2500 BC. It ended with the beginning of the Iron Age, 2000–1000 BC in the Near East, and 600 BC in Northern Europe. The transition between the Neolithic period and the Bronze Age was formerly termed the Chalcolithic period (copper-stone), when copper tools were used with stone tools. The term has gradually fallen out of favor because in some parts of the world, the Chalcolithic and Neolithic are coterminous at both ends. Brass, an alloy of copper and zinc, is of much more recent origin. It was known to the Greeks, but became a significant supplement to bronze during the Roman Empire.
Antiquity and Middle Ages


In Greece, copper was known by the name chalkos (χαλκός). It was an important resource for the Romans, Greeks and other ancient peoples. In Roman times, it was known as aes Cyprium, aes being the generic Latin term for copper alloys and Cyprium from Cyprus, where much copper was mined. The phrase was simplified to cuprum, hence the English copper. Aphrodite (Venusrin Rome)epresented copper in mythology and alchemy because of its lustrous beauty, its ancient use in producing mirrors; Cyprus was sacred to the goddess. The seven heavenly bodies known to the ancients were associated with the seven metals known in antiquity, and Venus was assigned to copper.
Britain's first used brass in about the 3rd or 2nd Century BC. In North America, copper mining began with marginal workings by Native Americans. Native copper is known to have been extracted from sites on Isle Royale with primitive stone tools between 800 and 1600. Copper metallurgy was flourishing in South America, particularly in Peru around 1000 AD; it proceeded at a much slower rate on other continents. Copper burial ornamentals from the 15th century have been uncovered, but the metal's commercial production did not start until the early 20th century.
The cultural role of copper has been important, particularly in currency. Romans in the 6th through 3rd centuries BC used copper lumps as money. At first, the copper itself was valued, but gradually the shape and look of the copper became more important. Julius Caesar had his own coins made from brass, while Octavianus Augustus Caesar's coins were made from Cu-Pb-Sn alloys. With an estimated annual output of around 15,000 t, Roman copper mining and smelting activities reached a scale unsurpassed until the time of the Industrial Revolution; the provinces most intensely mined were those of Hispania, Cyprus and in Central Europe.
The gates of the Temple of Jerusalem used Corinthian bronze treated with depletion gilding. The process was most prevalent in Alexandria, where alchemy is thought to have begun. In ancient India, copper was used in the holistic medical science Ayurveda for surgical instruments and other medical equipment. Ancient Egyptians (~2400 BC) used copper for sterilizing wounds and drinking water, and later to treat headaches, burns, and itching. The Baghdad Battery, with copper cylinders soldered to lead, dates back to 248 BC to AD 226 and resembles a galvanic cell, leading some people to believe this was the first battery; the hypothesis has not been verified.
Modern period

The Great Copper Mountain was a mine in Falun, Sweden, that operated from the 10th century to 1992. It satisfied two thirds of Europe's copper consumption in the 17th century and helped fund many of Sweden's wars during that time. It was referred to as the nation's treasury; Sweden had a copper backed currency.
Copper was used in roofing, currency, Renaissance sculpture, photographic technology known as the daguerreotype, the Statue of Liberty, and other structures. Copper plating and copper sheathing was widely used in the hulls of ships, of which the ships of Christopher Columbus were among the earliest. The Norddeutsche Affinerie in Hamburg was the first modern electroplating plant starting its production in 1876. The German scientist Gottfried Osann invented powder metallurgy in 1830 while determining the metal's atomic mass; around then it was discovered that the amount and type of alloying element (e.g., tin) to copper would affect bell tones. Flash smelting was developed by Outokumpu in Finland and first applied at Harjavalta in 1949; the energy-efficient process accounts for 50% of the world's primary copper production.
The Intergovernmental Council of Copper Exporting Countries, formed in 1967 by Chile, Peru, Zaire and Zambia, operates in the copper market as OPEC does for oil, though it never achieved the same influence, particularly because the second-largest producer, the United States, was never a member; it was dissolved in 1988.
Applications
See also: Copper in renewable energy
The major applications of copper are in electrical wires (60%), roofing and plumbing (20%), and industrial machinery (15%). Copper is mostly used as a pure metal, but when a higher hardness is required, it is alloyed with other elements (5% of total use) such as brass and bronze. For more than two centuries, copper paint has been used on boat hulls to control the growth of plants and shellfish. A small part of the copper supply is used for nutritional supplements and fungicides in agriculture. Machining of copper is possible, although alloys are preferred for good machinability characteristics in creating intricate parts.
Wire and cable
Main article: Copper wire and cableDespite competition from other materials, copper remains the preferred electrical conductor in nearly all categories of electrical wiring with the major exception being overhead electric power transmission where aluminium is often preferred. Copper wire is used in power generation, power transmission, power distribution, telecommunications, electronics circuitry, and countless types of electrical equipment. Electrical wiring is the most important market for the copper industry. This includes structural power wiring, power distribution cable, appliance wire, communications cable, automotive wire and cable, and magnet wire. Roughly half of all copper mined is used to manufacture electrical wire and cable conductors. Many electrical devices rely on copper wiring because of its multitude of inherent beneficial properties, such as its high electrical conductivity, tensile strength, ductility, creep (deformation) resistance, corrosion resistance, low thermal expansion, high thermal conductivity, solderability, and ease of installation.
For a short period from the late 1960s to the late 1970s, copper wiring was replaced by aluminum in many housing construction projects in America (see Aluminum wire for main article). The new wiring was implicated in a number of house fires and the industry returned to copper.
Electronics and related devices

Integrated circuits and printed circuit boards increasingly feature copper in place of aluminium because of its superior electrical conductivity (see Copper interconnect for main article); heat sinks and heat exchangers use copper as a result of its superior heat dissipation capacity to aluminium. Electromagnets, vacuum tubes, cathode ray tubes, and magnetrons in microwave ovens use copper, as do wave guides for microwave radiation.
Electric motors
Main article: Copper in energy efficient motorsCopper's superior conductivity enhances the efficiency of electrical motors. This is important because motors and motor-driven systems account for 43%-46% of all global electricity consumption and 69% of all electricity used by industry. Increasing the mass and cross section of copper in a coil increases the electrical energy efficiency of the motor. Copper motor rotors, a new technology designed for motor applications where energy savings are prime design objectives, are enabling general-purpose induction motors to meet and exceed National Electrical Manufacturers Association (NEMA) premium efficiency standards.
Architecture
Main article: Copper in architecture

Copper has been used since ancient times as a durable, corrosion resistant, and weatherproof architectural material. Roofs, flashings, rain gutters, downspouts, domes, spires, vaults, and doors have been made from copper for hundreds or thousands of years. Copper's architectural use has been expanded in modern times to include interior and exterior wall cladding, building expansion joints, radio frequency shielding, and antimicrobial indoor products, such as attractive handrails, bathroom fixtures, and counter tops. Some of copper's other important benefits as an architectural material include its low thermal movement, light weight, lightning protection, and its recyclability.
The metal's distinctive natural green patina has long been coveted by architects and designers. The final patina is a particularly durable layer that is highly resistant to atmospheric corrosion, thereby protecting the underlying metal against further weathering. It can be a mixture of carbonate and sulfate compounds in various amounts, depending upon environmental conditions such as sulfur-containing acid rain. Architectural copper and its alloys can also be 'finished' to embark a particular look, feel, and/or color. Finishes include mechanical surface treatments, chemical coloring, and coatings.
Copper has excellent brazing and soldering properties and can be welded; the best results are obtained with gas metal arc welding.
Antibiofouling applications
Main articles: Copper alloys in aquaculture and Copper sheathingCopper is biostatic, meaning bacteria will not grow on it. For this reason it has long been used to line parts of ships to protect against barnacles and mussels. It was originally used pure, but has since been superseded by Muntz metal. Similarly, as discussed in copper alloys in aquaculture, copper alloys have become important netting materials in the aquaculture industry because they are antimicrobial and prevent biofouling, even in extreme conditions and have strong structural and corrosion-resistant properties in marine environments.
Antimicrobial applications
Main articles: Antimicrobial properties of copper and Antimicrobial copper-alloy touch surfacesNumerous antimicrobial efficacy studies have been conducted in the past 10 years regarding copper's efficacy to destroy a wide range of bacteria, as well as influenza A virus, adenovirus, and fungi.
Copper-alloy touch surfaces have natural intrinsic properties to destroy a wide range of microorganisms (e.g., E. coli O157:H7, methicillin-resistant Staphylococcus aureus (MRSA), Staphylococcus, Clostridium difficile, influenza A virus, adenovirus, and fungi). Some 355 copper alloys were proven to kill more than 99.9% of disease-causing bacteria within just two hours when cleaned regularly. The United States Environmental Protection Agency (EPA) has approved the registrations of these copper alloys as "antimicrobial materials with public health benefits," which allows manufacturers to legally make claims as to the positive public health benefits of products made with registered antimicrobial copper alloys. In addition, the EPA has approved a long list of antimicrobial copper products made from these alloys, such as bedrails, handrails, over-bed tables, sinks, faucets, door knobs, toilet hardware, computer keyboards, health club equipment, shopping cart handles, etc. (for a comprehensive list of products, see: Antimicrobial copper-alloy touch surfaces#Approved products). Copper doorknobs are used by hospitals to reduce the transfer of disease, and Legionnaires' disease is suppressed by copper tubing in plumbing systems. Antimicrobial copper alloy products are now being installed in healthcare facilities in the U.K., Ireland, Japan, Korea, France, Denmark, and Brazil and in the subway transit system in Santiago, Chile, where copper-zinc alloy handrails will be installed in some 30 stations between 2011–2014.
Folk medicine
Copper is commonly used in jewelry, and folklore says that copper bracelets relieve arthritis symptoms.
In various studies, though, no difference is found between arthritis treated with a copper bracelet, magnetic bracelet, or placebo bracelet. As far as medical science is concerned, wearing copper has no known benefit, for any medical condition at all. A human being can have a dietary copper deficiency, but this is very rare, because copper is present in many common foods, including legumes (beans), grains, and nuts.
There is no evidence that copper can be absorbed through the skin. But if it were, it could lead to copper poisoning.
Compression clothing
More recently, some compression clothing has been sold with copper woven into it, with the same folk medicine claims being made. While compression clothing is a real treatment for some ailments, therefore the clothing may appear to work, the added copper may very well have no benefit beyond a placebo effect.
Other uses
Solutions of copper compounds are used as a wood preservative, particularly in treating original portion of structures during restoration of damage due to dry rot. Together with zinc, copper wires may be placed over non-conductive roofing materials to discourage the growth of moss. Textile fibers use copper to create antimicrobial protective fabrics,. It is used in musical instruments. Copper is commonly used as a base on which other metals such as nickel are electroplated.
Copper is one of three metals, along with lead and silver, used in a museum materials testing procedure called the Oddy test. In this procedure, copper is used to detect chlorides, oxides, and sulfur compounds.
Copper is used as the printing plate in etching, engraving and other forms of intaglio (printmaking) printmaking.
Copper oxide and carbonate are used to stain glass and in glassmaking and in ceramic glazes to impart green and brown colors.
Copper is the principal alloying metal in sterling silver and some gold alloys. It may also be used on its own, or as a constituent of brass, bronze, gilding metal and many other base metal alloys.
Degradation
Chromobacterium violaceum and Pseudomonas fluorescens can both mobilize solid copper, as a cyanide compound. The ericoid mycorrhizal fungi associated with Calluna, Erica and Vaccinium can grow in metalliferous soils containing copper. The ectomycorrhizal fungus Suillus luteus protects young pine trees from copper toxicity. A sample of the fungus Aspergillus niger was found growing from gold mining solution and was found to contain cyano metal complexes of metals such as gold, silver, copper iron and zinc. The fungus also plays a role in the solubilization of heavy metal sulfides.
Biological role
Main article: Copper in health
Copper proteins have diverse roles in biological electron transport and oxygen transportation, processes that exploit the easy interconversion of Cu(I) and Cu(II). The biological role for copper commenced with the appearance of oxygen in earth's atmosphere.
Copper is essential in the aerobic respiration of all eukaryotes. In mitochondria it is found in cytochrome c oxidase, which is the last protein in oxidative phosphorylation. Cytochrome c oxidase is the protein which binds the O2 between a copper and an iron, transferring 8 electrons to the O2 to reduce it to two molecules of water.
Copper is also found in many superoxide dismutases, proteins that catalyze the decomposition of superoxides, by converting it (by disproportionation) to oxygen and hydrogen peroxide:
- 2 HO2 → H2O2 + O2
The protein hemocyanin is the oxygen carrier in most mollusks and some arthropods such as the horseshoe crab (Limulus polyphemus). Because hemocyanin is blue, these organisms have blue blood, not the red blood found in organisms that rely on iron in hemoglobin for this purpose. Structurally related to hemocyanin are the laccases and tyrosinases. Instead of reversibly binding oxygen, these proteins hydroxylate substrates, illustrated by their role in the formation of lacquers.
Several copper proteins, such as the "blue copper proteins", do not interact directly with substrates, hence they are not enzymes. These proteins relay electrons by the process called electron transfer.
A unique tetranuclear copper center has been found in nitrous-oxide reductase.
Dietary needs
Copper is an essential trace element in plants and animals, but not some microorganisms. The human body contains copper at a level of about 1.4 to 2.1 mg per kg of body mass. Stated differently, the RDA for copper in normal healthy adults is quoted as 0.97 mg/day and as 3.0 mg/day. Copper is absorbed in the gut, then transported to the liver bound to albumin. After processing in the liver, copper is distributed to other tissues in a second phase. Copper transport here involves the protein ceruloplasmin, which carries the majority of copper in blood. Ceruloplasmin also carries copper that is excreted in milk, and is particularly well-absorbed as a copper source. Copper in the body normally undergoes enterohepatic circulation (about 5 mg a day, vs. about 1 mg per day absorbed in the diet and excreted from the body), and the body is able to excrete some excess copper, if needed, via bile, which carries some copper out of the liver that is not then reabsorbed by the intestine.
Copper-based disorders
Because of its role in facilitating iron uptake, copper deficiency can produce anemia-like symptoms, neutropenia, bone abnormalities, hypopigmentation, impaired growth, increased incidence of infections, osteoporosis, hyperthyroidism, and abnormalities in glucose and cholesterol metabolism. Conversely, Wilson's disease causes an accumulation of copper in body tissues.
Severe deficiency can be found by testing for low plasma or serum copper levels, low ceruloplasmin, and low red blood cell superoxide dismutase levels; these are not sensitive to marginal copper status. The "cytochrome c oxidase activity of leucocytes and platelets" has been stated as another factor in deficiency, but the results have not been confirmed by replication.
| NFPA 704 safety square | |
|---|---|
 |
Gram quantities of various copper salts have been taken in suicide attempts and produced acute copper toxicity in humans, possibly due to redox cycling and the generation of reactive oxygen species that damage DNA. Corresponding amounts of copper salts (30 mg/kg) are toxic in animals. A minimum dietary value for healthy growth in rabbits has been reported to be at least 3 ppm in the diet. However, higher concentrations of copper (100 ppm, 200 ppm, or 500 ppm) in the diet of rabbits may favorably influence feed conversion efficiency, growth rates, and carcass dressing percentages.
Chronic copper toxicity does not normally occur in humans because of transport systems that regulate absorption and excretion. Autosomal recessive mutations in copper transport proteins can disable these systems, leading to Wilson's disease with copper accumulation and cirrhosis of the liver in persons who have inherited two defective genes.
Elevated copper levels have also been linked to worsening symptoms of Alzheimer's disease.
Occupational exposure
In the US, the Occupational Safety and Health Administration (OSHA) has designated a permissible exposure limit (PEL) for copper dust and fumes in the workplace as a time-weighted average (TWA) of 1 mg/m. The National Institute for Occupational Safety and Health (NIOSH) has set a Recommended exposure limit (REL) of 1 mg/m, time-weighted average. The IDLH (immediately dangerous to life and health) value is 100 mg/m.
See also
- Electroplating
- Erosion corrosion of copper water tubes
- List of countries by copper production
- Metal theft
- Smelter
- Peak copper
- Category:Copper mining companies
References
- "Standard Atomic Weights: Copper". CIAAW. 1969.
- Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (4 May 2022). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- Cu(−2) have been observed as dimeric anions in La2Cu2In; see Changhoon Lee; Myung-Hwan Whangbo (2008). "Late transition metal anions acting as p-metal elements". Solid State Sciences. 10 (4): 444–449. Bibcode:2008SSSci..10..444K. doi:10.1016/j.solidstatesciences.2007.12.001.
- Moret, Marc-Etienne; Zhang, Limei; Peters, Jonas C. (2013). "A Polar Copper–Boron One-Electron σ-Bond". J. Am. Chem. Soc. 135 (10): 3792–3795. doi:10.1021/ja4006578. PMID 23418750.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 28. ISBN 978-0-08-037941-8.
- Lide, D. R., ed. (2005). "Magnetic susceptibility of the elements and inorganic compounds". CRC Handbook of Chemistry and Physics (PDF) (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5. Archived from the original (PDF) on 3 March 2011.
- Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- McHenry, Charles, ed. (1992). The New Encyclopedia Britannica. Vol. 3 (15 ed.). Chicago: Encyclopedia Britannica, Inc. p. 612. ISBN 085-229553-7.
- Encyclopaedia Britannica, 11th ed., vol. 7, p. 102.
- Johnson, MD PhD, Larry E., ed. (2008). "Copper". Merck Manual Home Health Handbook. Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. Retrieved 7 April 2013.
- "Copper in human health".
- ^ George L. Trigg; Edmund H. Immergut (1 November 1992). Encyclopedia of applied physics. Vol. 4: Combustion to Diamagnetism. VCH Publishers. pp. 267–272. ISBN 978-3-527-28126-8. Retrieved 2 May 2011.
- Smith, William F.; Hashemi, Javad (2003). Foundations of Materials Science and Engineering. McGraw-Hill Professional. p. 223. ISBN 0-07-292194-3.
{{cite book}}: Unknown parameter|last-author-amp=ignored (|name-list-style=suggested) (help) - ^ Hammond, C. R. (2004). The Elements, in Handbook of Chemistry and Physics (81st ed.). CRC press. ISBN 0-8493-0485-7.
- Resistance Welding Manufacturing Alliance (2003). Resistance Welding Manual (4th ed.). Resistance Welding Manufacturing Alliance. pp. 18–12. ISBN 0-9624382-0-0.
- Chambers, William; Chambers, Robert (1884). Chambers's Information for the People. Vol. L (5th ed.). W. & R. Chambers. p. 312. ISBN 0-665-46912-8.
- "Galvanic Corrosion". Corrosion Doctors. Retrieved 29 April 2011.
- ^ Grieken, Rene van; Janssens, Koen (27 September 2005). Cultural Heritage Conservation and Environmental Impact Assessment by Non-Destructive Testing and Micro-Analysis. CRC Press. p. 197. ISBN 9780203970782.
- "Copper.org: Education: Statue of Liberty: Reclothing the First Lady of Metals – Repair Concerns". Copper.org. Retrieved 11 April 2011.
- Rickett, B. I.; Payer, J. H. (1995). "Composition of Copper Tarnish Products Formed in Moist Air with Trace Levels of Pollutant Gas: Hydrogen Sulfide and Sulfur Dioxide/Hydrogen Sulfide". Journal of the Electrochemical Society. 142 (11): 3723–3728. doi:10.1149/1.2048404.
- ^ Audi, G; Bersillon, O.; Blachot, J.; Wapstra, A.H. (2003). "Nubase2003 Evaluation of Nuclear and Decay Properties". Nuclear Physics A. 729. Atomic Mass Data Center: 3. Bibcode:2003NuPhA.729....3A. doi:10.1016/j.nuclphysa.2003.11.001.
- "Interactive Chart of Nuclides". National Nuclear Data Center. Retrieved 8 April 2011.
- Okazawad, Hidehiko; Yonekura, Yoshiharu; Fujibayashi, Yasuhisa; Nishizawa, Sadahiko; Magata, Yasuhiro; Ishizu, Koichi; Tanaka, Fumiko; Tsuchida, Tatsuro; Tamaki, Nagara; Konishi, Junji (1994). "Clinical Application and Quantitative Evaluation of Generator-Produced Copper-62-PTSM as a Brain Perfusion Tracer for PET" (PDF). Journal of Nuclear Medicine. 35 (12): 1910–1915. PMID 7989968.
- Romano, Donatella; Matteucci, Fransesca (2007). "Contrasting copper evolution in ω Centauri and the Milky Way". Monthly Notices of the Royal Astronomical Society: Letters. 378 (1): L59 – L63. arXiv:astro-ph/0703760. Bibcode:2007MNRAS.378L..59R. doi:10.1111/j.1745-3933.2007.00320.x.
- ^ Emsley, John (11 August 2003). Nature's building blocks: an A-Z guide to the elements. Oxford University Press. pp. 121–125. ISBN 978-0-19-850340-8. Retrieved 2 May 2011.
- Rickwood, P. C. (1981). "The largest crystals" (PDF). American Mineralogist. 66: 885.
- Randazzo, Ryan (19 June 2011). "A new method to harvest copper". Azcentral.com. Retrieved 25 April 2014.
- Gordon, R. B.; Bertram, M.; Graedel, T. E. (2006). "Metal stocks and sustainability". PNAS. 103 (5): 1209–1214. Bibcode:2006PNAS..103.1209G. doi:10.1073/pnas.0509498103. PMC 1360560. PMID 16432205.
- ^ Leonard, Andrew (2 March 2006). "Peak copper?". Salon – How the World Works. Retrieved 23 March 2008.
- Brown, Lester (2006). Plan B 2.0: Rescuing a Planet Under Stress and a Civilization in Trouble. New York: W.W. Norton. p. 109. ISBN 0-393-32831-7.
- Schmitz, Christopher (1986). "The Rise of Big Business in the World, Copper Industry 1870–1930". Economic History Review. 2. 39 (3): 392–410. doi:10.1111/j.1468-0289.1986.tb00411.x. JSTOR 2596347.
- "Copper Trends: Live Metal Spot Prices".
- Ackerman, R. (2 April 2009). "A Bottom In Sight For Copper". Forbes.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Watling, H. R. (2006). "The bioleaching of sulphide minerals with emphasis on copper sulphides — A review" (PDF). Hydrometallurgy. 84 (1, 2): 81–108. doi:10.1016/j.hydromet.2006.05.001.
- Samans, Carl (1949). Engineering metals and their alloys. New York: Macmillan. OCLC 716492542.
- "International Copper Association".
- "Overview of Recycled Copper" Copper.org. Copper.org (25 August 2010). Retrieved on 8 November 2011.
- "Gold Jewellery Alloys". World Gold Council. Retrieved 6 June 2009.
- Corrosion Tests and Standards. ASTM International. p. 368.
- Balver Zinn Solder Sn97Cu3. (PDF) . balverzinn.com. Retrieved on 8 November 2011.
- ^ Holleman, A. F.; Wiberg, N. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 978-0-12-352651-9.
- ^ Wiley-Vch (2 April 2007). "Nonsystematic (Contact) Fungicides". Ullmann's Agrochemicals. p. 623. ISBN 978-3-527-31604-5.
- Ralph L. Shriner, Christine K. F. Hermann, Terence C. Morrill, David Y. Curtin, Reynold C. Fuson "The Systematic Identification of Organic Compounds" 8th edition, J. Wiley, Hoboken. ISBN 0-471-21503-1
- Kay Saalwächter, Walther Burchard, Peter Klüfers, G. Kettenbach, and Peter Mayer, Dieter Klemm, Saran Dugarmaa "Cellulose Solutions in Water Containing Metal Complexes" Macromolecules 2000, 33, 4094–4107. doi:10.1021/ma991893m
- "Modern Organocopper Chemistry" Norbert Krause, Ed., Wiley-VCH, Weinheim, 2002. ISBN 978-3-527-29773-3.
- Berná, José; Goldup, Stephen; Lee, Ai-Lan; Leigh, David; Symes, Mark; Teobaldi, Gilberto; Zerbetto, Fransesco (26 May 2008). "Cadiot–Chodkiewicz Active Template Synthesis of Rotaxanes and Switchable Molecular Shuttles with Weak Intercomponent Interactions". Angewandte Chemie. 120 (23): 4464–4468. doi:10.1002/ange.200800891.
- Rafael Chinchilla; Carmen Nájera (2007). "The Sonogashira Reaction: A Booming Methodology in Synthetic Organic Chemistry". Chemical Reviews. 107 (3): 874–922. doi:10.1021/cr050992x. PMID 17305399.
{{cite journal}}: Unknown parameter|last-author-amp=ignored (|name-list-style=suggested) (help) - "An Addition of an Ethylcopper Complex to 1-Octyne: (E)-5-Ethyl-1,4-Undecadiene" (PDF). Organic Syntheses. 64: 1. 1986. doi:10.15227/orgsyn.064.0001.
- Kharasch, M. S.; Tawney, P. O. (1941). "Factors Determining the Course and Mechanisms of Grignard Reactions. II. The Effect of Metallic Compounds on the Reaction between Isophorone and Methylmagnesium Bromide". Journal of the American Chemical Society. 63 (9): 2308–2316. doi:10.1021/ja01854a005.
- Imai, Sadako; Fujisawa, Kiyoshi; Kobayashi, Takako; Shirasawa, Nobuhiko; Fujii, Hiroshi; Yoshimura, Tetsuhiko; Kitajima, Nobumasa; Moro-oka, Yoshihiko (1998). "Cu NMR Study of Copper(I) Carbonyl Complexes with Various Hydrotris(pyrazolyl)borates: Correlation between 63Cu Chemical Shifts and CO Stretching Vibrations". Inorg. Chem. 37 (12): 3066–3070. doi:10.1021/ic970138r.
- G. Brauer, ed. (1963). "Potassium Cuprate (III)". Handbook of Preparative Inorganic Chemistry. Vol. 1 (2nd ed.). NY: Academic Press. p. 1015.
- Schwesinger, Reinhard; Link, Reinhard; Wenzl, Peter; Kossek, Sebastian (2006). "Anhydrous phosphazenium fluorides as sources for extremely reactive fluoride ions in solution". Chemistry – A European Journal. 12 (2): 438. doi:10.1002/chem.200500838.
- Lewis, E. A.; Tolman, W. B. (2004). "Reactivity of Dioxygen-Copper Systems". Chemical Reviews. 104 (2): 1047–1076. doi:10.1021/cr020633r. PMID 14871149.
- McDonald, M. R.; Fredericks, F. C.; Margerum, D. W. (1997). "Characterization of Copper(III)-Tetrapeptide Complexes with Histidine as the Third Residue". Inorganic Chemistry. 36 (14): 3119–3124. doi:10.1021/ic9608713. PMID 11669966.
- ^ "CSA – Discovery Guides, A Brief History of Copper". Csa.com. Retrieved 12 September 2008.
- Rayner W. Hesse (2007). Jewelrymaking through History: an Encyclopedia. Greenwood Publishing Group. p. 56. ISBN 0-313-33507-9.No primary source is given in that book.
- "Copper". Elements.vanderkrogt.net. Retrieved 12 September 2008.
- Renfrew, Colin (1990). Before civilization: the radiocarbon revolution and prehistoric Europe. Penguin. ISBN 978-0-14-013642-5. Retrieved 21 December 2011.
- Cowen, R. "Essays on Geology, History, and People". Retrieved 7 July 2009.
- Timberlake, S.; Prag A.J.N.W. (2005). The Archaeology of Alderley Edge: Survey, excavation and experiment in an ancient mining landscape. Oxford: John and Erica Hedges Ltd. p. 396.
{{cite book}}: Unknown parameter|last-author-amp=ignored (|name-list-style=suggested) (help) - ^ "CSA – Discovery Guides, A Brief History of Copper". CSA Discovery Guides. Retrieved 29 April 2011.
- Pleger, Thomas C. "A Brief Introduction to the Old Copper Complex of the Western Great Lakes: 4000–1000 BC", Proceedings of the Twenty-seventh Annual Meeting of the Forest History Association of Wisconsin, Oconto, Wisconsin, 5 October 2002, pp. 10–18.
- Emerson, Thomas E. and McElrath, Dale L. Archaic Societies: Diversity and Complexity Across the Midcontinent, SUNY Press, 2009 ISBN 1-4384-2701-8.
- Radivojević, Miljana; Rehren, Thilo (December 2013). "Tainted ores and the rise of tin bronzes in Eurasia, c. 6500 years ago". Antiquity Publications Ltd.
- ^ McNeil, Ian (2002). Encyclopaedia of the History of Technology. London ; New York: Routledge. pp. 13, 48–66. ISBN 0-203-19211-7.
- Rickard, T. A. (1932). "The Nomenclature of Copper and its Alloys". Journal of the Royal Anthropological Institute. 62. Royal Anthropological Institute: 281. doi:10.2307/2843960. JSTOR 2843960.
- Martin, Susan R. (1995). "The State of Our Knowledge About Ancient Copper Mining in Michigan". The Michigan Archaeologist. 41 (2–3): 119.
- Hong, S.; Candelone, J.-P.; Patterson, C. C.; Boutron, C. F. (1996). "History of Ancient Copper Smelting Pollution During Roman and Medieval Times Recorded in Greenland Ice". Science. 272 (5259): 246–249 (247f.). Bibcode:1996Sci...272..246H. doi:10.1126/science.272.5259.246.
- de Callataÿ, François (2005). "The Graeco-Roman Economy in the Super Long-Run: Lead, Copper, and Shipwrecks". Journal of Roman Archaeology. 18: 361–372 (366–369).
- Savenije, Tom J.; Warman, John M.; Barentsen, Helma M.; van Dijk, Marinus; Zuilhof, Han; Sudhölter, Ernst J. R. (2000). "Corinthian Bronze and the Gold of the Alchemists" (PDF). Macromolecules. 33 (2): 60. Bibcode:2000MaMol..33...60S. doi:10.1021/ma9904870.
- "World Mysteries – Strange Artifacts, Baghdad Battery". World-Mysteries.com. Retrieved 22 April 2011.
- Lynch, Martin (15 April 2004). Mining in World History. p. 60. ISBN 978-1-86189-173-0.
- "Gold: prices, facts, figures and research: A brief history of money". Retrieved 22 April 2011.
- "Copper History". Retrieved 4 September 2008.
- Stelter, M.; Bombach, H. (2004). "Process Optimization in Copper Electrorefining". Advanced Engineering Materials. 6 (7): 558. doi:10.1002/adem.200400403.
- "Outokumpu Flash Smelting" (PDF). Outokumpu. p. 2. Archived from the original (PDF) on 24 July 2011.
- Karen A. Mingst (1976). "Cooperation or illusion: an examination of the intergovernmental council of copper exporting countries". International Organization. 30 (2): 263–287. doi:10.1017/S0020818300018270.
- "Is Copper Bottom Paint Sinking? - BoatUS Magazine". http://www.boatus.com. Retrieved 3 June 2016.
{{cite web}}: External link in|website= - "Copper". American Elements. 2008. Retrieved 12 July 2008.
- Pops, Horace, 2008, Processing of wire from antiquity to the future, Wire Journal International, June, pp 58–66
- The Metallurgy of Copper Wire, http://www.litz-wire.com/pdf%20files/Metallurgy_Copper_Wire.pdf
- Joseph, Günter, 1999, Copper: Its Trade, Manufacture, Use, and Environmental Status, edited by Kundig, Konrad J.A., ASM International, pps. 141–192 and pps. 331–375.
- "Copper, Chemical Element – Overview, Discovery and naming, Physical properties, Chemical properties, Occurrence in nature, Isotopes". Chemistryexplained.com. Retrieved 16 October 2012.
- Joseph, Günter, 1999, Copper: Its Trade, Manufacture, Use, and Environmental Status, edited by Kundig, Konrad J.A., ASM International, p.348
- "The Requested Page has moved". www.heimer.com. Retrieved 3 June 2016.
- "Electrical Wiring FAQ (Part 2 of 2)Section - Aluminum wiring". www.faqs.org. Retrieved 3 June 2016.
- "Accelerator: Waveguides (SLAC VVC)". SLAC Virtual Visitor Center. Retrieved 29 April 2011.
- IE3 energy-saving motors, Engineer Live, http://www.engineerlive.com/Design-Engineer/Motors_and_Drives/IE3_energy-saving_motors/22687/
- Energy‐efficiency policy opportunities for electric motor‐driven systems, International Energy Agency, 2011 Working Paper in the Energy Efficiency Series, by Paul Waide and Conrad U. Brunner, OECD/IEA 2011
- Fuchsloch, J. and E.F. Brush, (2007), "Systematic Design Approach for a New Series of Ultra‐NEMA Premium Copper Rotor Motors", in EEMODS 2007 Conference Proceedings, 10‐15 June,Beijing.
- Copper motor rotor project; Copper Development Association; http://www.copper.org/applications/electrical/motor-rotor
- NEMA Premium Motors, The Association of Electrical Equipment and Medical Imaging Manufacturers; http://www.nema.org/gov/energy/efficiency/premium/
- Seale, Wayne (2007). The role of copper, brass, and bronze in architecture and design; Metal Architecture, May 2007
- Copper roofing in detail; Copper in Architecture; Copper Development Association, U.K., www.cda.org.uk/arch
- Architecture, European Copper Institute; http://eurocopper.org/copper/copper-architecture.html
- Kronborg completed; Agency for Palaces and Cultural Properties, København, http://www.slke.dk/en/slotteoghaver/slotte/kronborg/kronborgshistorie/kronborgfaerdigbygget.aspx?highlight=copper
- Berg, Jan. "Why did we paint the library's roof?". Archived from the original on 25 June 2007. Retrieved 20 September 2007.
- Architectural considerations; Copper in Architecture Design Handbook, http://www.copper.org/applications/architecture/arch_dhb/fundamentals/arch_considerations.htm
- Peters, Larry E. (2004). Preventing corrosion on copper roofing systems; Professional Roofing, October 2004, http://www.professionalroofing.net
- Oxidation Reaction: Why is the Statue of Liberty Blue-Green? Engage Students in Engineering; www.EngageEngineering.org; Chun Wu, Ph.D., Mount Marty College; Funded by the National Science Foundation (NSF) under Grant No. 083306. http://www.wepanknowledgecenter.org/c/document_library/get_file?folderId=517&name=DLFE-2454.pdf
- Fitzgerald, K.P.; Nairn, J.; Atrens, A. (1998). "The chemistry of copper patination". Corrosion Science. 40 (12): 2029–50. doi:10.1016/S0010-938X(98)00093-6.
- Application Areas: Architecture – Finishes – patina; http://www.copper.org/applications/architecture/finishes.html
- Glossary of copper terms, Copper Development Association (UK): http://www.copperinfo.co.uk/resources/glossary.shtml
- Finishes – natural weathering; Copper in Architecture Design Handbook, Copper Development Association Inc., http://www.copper.org/applications/architecture/arch_dhb/finishes/finishes.html
- Davis, Joseph R. (2001). Copper and Copper Alloys. ASM International. pp. 3–6, 266. ISBN 0-87170-726-8.
- Edding, Mario E., Flores, Hector, and Miranda, Claudio, (1995), Experimental Usage of Copper-Nickel Alloy Mesh in Mariculture. Part 1: Feasibility of usage in a temperate zone; Part 2: Demonstration of usage in a cold zone; Final report to the International Copper Association Ltd.
- Corrosion Behaviour of Copper Alloys used in Marine Aquaculture. (PDF) . copper.org. Retrieved on 8 November 2011.
- ^ Copper Touch Surfaces. Copper Touch Surfaces. Retrieved on 8 November 2011.
- ^ EPA registers copper-containing alloy products, May 2008
- Biurrun, Amaya; Caballero, Luis; Pelaz, Carmen; León, Elena; Gago, Alberto (1999). "Treatment of a Legionella pneumophila‐Colonized Water Distribution System Using Copper‐Silver Ionization and Continuous Chlorination". Infection Control and Hospital Epidemiology. 20 (6): 426–428. doi:10.1086/501645. JSTOR 30141645. PMID 10395146.
- Chilean subway protected with Antimicrobial Copper – Rail News from. rail.co. Retrieved on 8 November 2011.
- Codelco to provide antimicrobial copper for new metro lines (Chile). Construpages.com.ve. Retrieved on 8 November 2011.
- PR 811 Chilean Subway Installs Antimicrobial Copper. (PDF). antimicrobialcopper.com. Retrieved on 8 November 2011.
- Walker, WR; Keats, DM (1976). "An investigation of the therapeutic value of the 'copper bracelet'-dermal assimilation of copper in arthritic/rheumatoid conditions". Agents and actions. 6 (4): 454–9. PMID 961545.
- The Daily Mail:
http://www.dailymail.co.uk/health/article-1221015/Copper-bracelet-arthritis-cure-myth-say-scientists-casting-doubt-multi-million-pound-alternative-healthcare-industry.html Copper bracelet arthritis cure is a myth, say scientists] - National Institutes of Health (NIH):
PMID 19942103
No difference was observed between devices in terms of their effects on pain as measured by the primary outcome measure (WOMAC A), the PRI and the VAS. Similar results were obtained for stiffness (WOMAC B), physical function (WOMAC C), and medication use. Further analyses of the PRI subscales revealed a statistically significant difference between devices (P=0.025), which favoured the experimental device. Participants reported lower sensory pain after wearing the standard magnetic wrist strap, than when wearing control devices. However, no adjustment was made for multiple testing. - Richmond, Stewart J.; Brown, Sally R.; Campion, Peter D.; Porter, Amanda J.L.; Moffett, Jennifer A. Klaber; Jackson, David A.; Featherstone, Valerie A.; Taylor, Andrew J. (2009). "Therapeutic effects of magnetic and copper bracelets in osteoarthritis: A randomised placebo-controlled crossover trial". Complementary Therapies in Medicine. 17 (5–6): 249–256. doi:10.1016/j.ctim.2009.07.002. ISSN 0965-2299.
- University of Arkansas for Medical Sciences:
Can wearing a copper bracelet cure arthritis?
According to the Center for Hand and Upper Extremity Surgery at UAMS, copper deficiency is extremely rare and most regular diets provide enough copper to meet the daily requirements. Copper is a component of some of the normal cellular enzymes in most mineral rich foods, such as vegetables, potatoes, legumes (beans and peas), nuts (peanuts and pecans), grains (wheat and rye) and fruits. Supplementation is only needed in patients with serious medical conditions that affect their gastrointestinal tract and impair their ability to absorb nutrients. - University of Arkansas for Medical Sciences:
Find the Truth Behind Medical Myths
While it's never been proven that copper can copper be absorbed through the skin by wearing a bracelet, research has shown that excessive copper can result in poisoning, causing vomiting and, in severe cases, liver damage. -
Truth in Advertising
Tommie Copper
So it seems possible that copper-infused compression clothing could help you recover from a tough workout, and it's also possible it could have some anti-bacterial properties in clothes. But as for the claims in the infomercial about relieving joint pain and helping with everyday aches — any relief from copper-compression seems more likely to be a placebo effect than anything else. Think carefully before shelling out for Tommie Copper. - "Copper and Cupron". Cupron.
- Ergowear, Copper antimicrobial yarn technology used in male underwear
- ^ Geoffrey Michael Gadd (March 2010). "Metals, minerals and microbes: geomicrobiology and bioremediation". Microbiology. 156 (3): 609–643. doi:10.1099/mic.0.037143-0. PMID 20019082.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Harbhajan Singh (17 November 2006). Mycoremediation: Fungal Bioremediation. p. 509. ISBN 9780470050583.
- Vest, Katherine E.; Hashemi, Hayaa F.; Cobine, Paul A. (2013). "Chapter 13 The Copper Metallome in Eukaryotic Cells". In Banci, Lucia (ed.). Metallomics and the Cell. Metal Ions in Life Sciences. Vol. 12. Springer. doi:10.1007/978-94-007-5561-10_12. ISBN 978-94-007-5560-4. electronic-book ISBN 978-94-007-5561-1 ISSN 1559-0836 electronic-ISSN 1868-0402
- Vest, Katherine E.; Hashemi, Hayaa F.; Cobine, Paul A. (2013). "Chapter 12 The Copper Metallome in Prokaryotic Cells". In Banci, Lucia (ed.). Metallomics and the Cell. Metal Ions in Life Sciences. Vol. 12. Springer. doi:10.1007/978-94-007-5561-10_13. ISBN 978-94-007-5560-4. electronic-book ISBN 978-94-007-5561-1 ISSN 1559-0836 electronic-ISSN 1868-0402
- Yee, Gereon M.; Tolman, William B. (2015). "Chapter 5, Section 4 Dioxygen Activation by Copper Complexes". In Peter M.H. Kroneck and Martha E. Sosa Torres (ed.). Sustaining Life on Planet Earth: Metalloenzymes Mastering Dioxygen and Other Chewy Gases. Metal Ions in Life Sciences. Vol. 15. Springer. pp. 175–192. doi:10.1007/978-3-319-12415-5_5.
- ^ S. J. Lippard, J. M. Berg "Principles of bioinorganic chemistry" University Science Books: Mill Valley, CA; 1994. ISBN 0-935702-73-3.
- Decker, H.; Terwilliger, N. (2000). "COPs and Robbers: Putative evolution of copper oxygen-binding proteins". Journal of Experimental Biology. 203 (Pt 12): 1777–1782. PMID 10821735.
{{cite journal}}: Unknown parameter|last-author-amp=ignored (|name-list-style=suggested) (help) - "Fun facts". Horseshoe crab. University of Delaware. Retrieved 13 July 2008.
- Schneider, Lisa K.; Wüst, Anja; Pomowski, Anja; Zhang, Lin; Einsle, Oliver (2014). "Chapter 8. No Laughing Matter: The Unmaking of the Greenhouse Gas Dinitrogen Monoxide by Nitrous Oxide Reductase". In Peter M.H. Kroneck; Martha E. Sosa Torres (eds.). The Metal-Driven Biogeochemistry of Gaseous Compounds in the Environment. Metal Ions in Life Sciences. Vol. 14. Springer. pp. 177–210. doi:10.1007/978-94-017-9269-1_8.
- ^ "Amount of copper in the normal human body, and other nutritional copper facts". Retrieved 3 April 2009.
- Copper. In: Recommended Dietary Allowances. Washington, D.C.: National Research Council, Food Nutrition Board, NRC/NAS. 1980. pp. 151–154.
- Adelstein, S. J.; Vallee, B. L. (1961). "Copper metabolism in man". New England Journal of Medicine. 265 (18): 892–897. doi:10.1056/NEJM196111022651806.
- M C Linder; Wooten, L; Cerveza, P; Cotton, S; Shulze, R; Lomeli, N (1 May 1998). "Copper transport". The American Journal of Clinical Nutrition. 67 (5): 965S – 971S. PMID 9587137.
- Frieden, E; Hsieh, HS (1976). "Ceruloplasmin: The copper transport protein with essential oxidase activity". Advances in Enzymology and Related Areas of Molecular Biology. Advances in Enzymology - and Related Areas of Molecular Biology. 44: 187–236. doi:10.1002/9780470122891.ch6. ISBN 9780470122891. JSTOR 20170553. PMID 775938.
- S. S. Percival; Harris, ED (1 January 1990). "Copper transport from ceruloplasmin: Characterization of the cellular uptake mechanism". American Journal of Physiology. Cell Physiology. 258 (1): C140–6. PMID 2301561.
- Bonham, Maxine; O'Connor, Jacqueline M.; Hannigan, Bernadette M.; Strain, J. J. (2002). "The immune system as a physiological indicator of marginal copper status?". British Journal of Nutrition. 87 (5): 393–403. doi:10.1079/BJN2002558. PMID 12010579.
- Li, Yunbo; Trush, Michael; Yager, James (1994). "DNA damage caused by reactive oxygen species originating from a copper-dependent oxidation of the 2-hydroxy catechol of estradiol". Carcinogenesis. 15 (7): 1421–1427. doi:10.1093/carcin/15.7.1421. PMID 8033320.
- Gordon, Starkebaum; John, M. Harlan (April 1986). "Endothelial cell injury due to copper-catalyzed hydrogen peroxide generation from homocysteine". J. Clin. Invest. 77 (4): 1370–6. doi:10.1172/JCI112442. PMC 424498. PMID 3514679.
- "Pesticide Information Profile for Copper Sulfate". Cornell University. Retrieved 10 July 2008.
- Hunt, Charles E.; William W. Carlton (1965). "Cardiovascular Lesions Associated with Experimental Copper Deficiency in the Rabbit". Journal of Nutrition. 87 (4): 385–394. PMID 5841854.
{{cite journal}}: Unknown parameter|last-author-amp=ignored (|name-list-style=suggested) (help) - Ayyat M.S.; Marai I.F.M.; Alazab A.M. (1995). "Copper-Protein Nutrition of New Zealand White Rabbits under Egyptian Conditions". World Rabbit Science. 3 (3): 113–118. doi:10.4995/wrs.1995.249.
- Brewer GJ. Copper excess, zinc deficiency, and cognition loss in Alzheimer's disease. BioFactors (Oxford, England). March 2012;38(2):107–113. doi:10.1002/biof.1005. PMID 22438177.
- "Copper: Alzheimer's Disease". Examine.com. Retrieved 21 June 2015.
- NIOSH Pocket Guide to Chemical Hazards. "#0150". National Institute for Occupational Safety and Health (NIOSH).
Notes
 |
 |
 |
 |
| in pure water, or acidic or alkali conditions. Copper in neutral water is more noble than hydrogen. | in water containing sulfide | in 10 M ammonia solution | in a chloride solution |
Further reading
- Massaro, Edward J., ed. (2002). Handbook of Copper Pharmacology and Toxicology. Humana Press. ISBN 0-89603-943-9.
- "Copper: Technology & Competitiveness (Summary) Chapter 6: Copper Production Technology" (PDF). Office of Technology Assessment. 2005.
- Current Medicinal Chemistry, Volume 12, Number 10, May 2005, pp. 1161–1208(48) Metals, Toxicity and Oxidative Stress
- William D. Callister (2003). Materials Science and Engineering: an Introduction (6th ed.). Wiley, New York. Table 6.1, p. 137. ISBN 0-471-73696-1.
- Material: Copper (Cu), bulk, MEMS and Nanotechnology Clearinghouse.
- Kim BE; Nevitt T; Thiele DJ (2008). "Mechanisms for copper acquisition, distribution and regulation". Nat. Chem. Biol. 4 (3): 176–85. doi:10.1038/nchembio.72. PMID 18277979.
- Copper transport disorders: an Instant insight from the Royal Society of Chemistry
External links
- Copper at The Periodic Table of Videos (University of Nottingham)
- National Pollutant Inventory – Copper and compounds fact sheet
- Copper Resource Page. Includes several PDF files detailing the material properties of various kinds of copper, as well as various guides and tools for the copper industry.
- CDC – NIOSH Pocket Guide to Chemical Hazards – Copper (dusts and mists)
- CDC – NIOSH Pocket Guide to Chemical Hazards – Copper fume
- The Copper Development Association has an extensive site of properties and uses of copper; it also maintains a web site dedicated to brass, a copper alloy.
- The Third Millennium Online page on Copper
- Price history of copper, according to the IMF
| Periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Template:Chemical elements named after places
| Copper compounds | |
|---|---|
| Cu(0,I) | |
| Cu(I) | |
| Cu(I,II) | |
| Cu(II) | |
| Cu(III) | |
| Cu(IV) | |
| Jewellery | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Forms | |||||||||||||
| Making |
| ||||||||||||
| Materials |
| ||||||||||||
| Terms |
| ||||||||||||
| |||||||||||||


