| Revision as of 02:38, 5 July 2016 editHeadbomb (talk | contribs)Edit filter managers, Autopatrolled, Extended confirmed users, Page movers, File movers, New page reviewers, Pending changes reviewers, Rollbackers, Template editors455,045 editsm clean up, replaced: Journal of nuclear medicine → Journal of Nuclear Medicine (2) using AWB← Previous edit | Revision as of 00:50, 9 July 2016 edit undoSfarney (talk | contribs)Extended confirmed users3,974 edits Correct sp. of pertechnetate; wikilinks; copyedit through Fission product for commercial useTags: nowiki added Visual editNext edit → | ||
| Line 1: | Line 1: | ||
| {{pp-move-indef}} | {{pp-move-indef}} | ||
| {{Infobox technetium}} | {{Infobox technetium}} | ||
| '''Technetium''' ({{IPAc-en|t|ɛ|k|'|n|iː|ʃ|i|ə|m}}) is a ] with symbol '''Tc''' and ] 43. |
'''Technetium''' ({{IPAc-en|t|ɛ|k|'|n|iː|ʃ|i|ə|m}}) is a ] with symbol '''Tc''' and ] 43. It is the lightest element of which all isotopes are ]; none are ]. Only one other element, ], is followed (in the periodic table) by elements with stable isotopes. Nearly all technetium is produced synthetically, and only minute amounts are found in the Earth's crust. Naturally occurring technetium is a spontaneous ] in ] or the product of ] in ] ores. The chemical properties of this silvery gray, crystalline ] are intermediate between ] and ]. | ||
| Many of technetium's properties were predicted by ] before the element was discovered. Mendeleev noted a gap in his ] and gave the undiscovered element the provisional name '']'' (''Em''). In 1937, technetium (specifically the ] isotope) became the first predominantly artificial element to be produced, hence its name (from the Greek {{lang|el|''τεχνητός''}}, meaning "artificial", + '']''). | Many of technetium's properties were predicted by ] before the element was discovered. Mendeleev noted a gap in his ] and gave the undiscovered element the provisional name '']'' (''Em''). In 1937, technetium (specifically the ] isotope) became the first predominantly artificial element to be produced, hence its name (from the Greek {{lang|el|''τεχνητός''}}, meaning "artificial", + '']''). | ||
| Its short-lived ]-emitting ]—]—is used in ] for a wide variety of diagnostic tests. Technetium-99 is used as a gamma-ray-free source of ]s. Long-lived ] produced commercially are by-products of ] of ] in ]s and are extracted from ]. Because no isotope of technetium has a ] longer than 4.2 million years (]), |
Its short-lived ]-emitting ]—]—is used in ] for a wide variety of diagnostic tests. Technetium-99 is used as a gamma-ray-free source of ]s. Long-lived ] produced commercially are by-products of ] of ] in ]s and are extracted from ]. Because no isotope of technetium has a ] longer than 4.2 million years (]), the 1952 detection of technetium in ]s, which are billions of years old, helped to prove that stars can produce heavier elements. | ||
| ==History== | ==History== | ||
| ===Search for element 43=== | ===Search for element 43=== | ||
| From the 1860s through 1871, early forms of the periodic table proposed by Dmitri Mendeleev contained a gap between ] (element 42) and ] (element 44). In 1871, Mendeleev predicted this missing element would occupy the empty place below ] and |
From the 1860s through 1871, early forms of the periodic table proposed by Dmitri Mendeleev contained a gap between ] (element 42) and ] (element 44). In 1871, Mendeleev predicted this missing element would occupy the empty place below ] and have similar chemical properties. Mendeleev gave it the provisional name ''ekamanganese'' (from ''eka''-, the ] word for ''one)'' because the predicted element was one place down from the known element manganese.<ref>{{cite journal|doi = 10.1007/BF00837634|title = Technetium, the missing element|date = 1996|last = Jonge|first = F. A. A.|journal = European Journal of Nuclear Medicine|volume = 23|pages = 336–44|pmid = 8599967|last2 = Pauwels|first2 = EK|issue = 3}}</ref> | ||
| ===Early mis-identifications=== | ===Early mis-identifications=== | ||
| Line 53: | Line 53: | ||
| ===Unreproducible results=== | ===Unreproducible results=== | ||
| German chemists ], ], and ] reported the discovery of element 75 and element 43 in 1925, and named element 43 '']'' (after ] in eastern ], now in ], the region where Walter Noddack's family originated).<ref name="multidict"/> The group bombarded ] with a beam of ]s and deduced element 43 was present by examining ] diffraction ]s.<ref name="Emsley2001p423">{{harvnb|Emsley|2001|p=423}}</ref> The ] of the X-rays produced is related to the atomic number by a formula derived by ] in 1913. The team claimed to detect a faint X-ray signal at a wavelength produced by element 43. Later experimenters could not replicate the discovery, and it was dismissed as an error for many years.<ref name="armstrong">{{cite journal|last=Armstrong |first=J. T.|url=http://pubs.acs.org/cen/80th/technetium.html | doi = 10.1021/cen-v081n036.p110|title=Technetium|publisher=Chemical & Engineering News|date=2003|accessdate = 2009-11-11}}</ref><ref>{{cite news| first = K. A.|last = Nies|url = http://www.hypatiamaze.org/ida/tacke.html|title = Ida Tacke and the warfare behind the discovery of fission|date = 2001| accessdate = 2009-05-05}}</ref> Still, in 1933, a series of articles on the discovery of elements quoted the name ''masurium'' for element 43.<ref>{{cite journal|title = The discovery of the elements. XX. Recently discovered elements|last = Weeks|first = M. E.|journal = Journal of Chemical Education|date = 1933|pages = 161–170|doi = 10.1021/ed010p161|volume = 10|issue = 3|bibcode = 1933JChEd..10..161W }}</ref><ref group=note>In 1998 John T. Armstrong of the ] ran "computer simulations" of the 1925 experiments and obtained results quite close to those reported by the Noddack team. "Using first-principles X-ray-emission spectral-generation algorithms developed at NIST, I simulated the X-ray spectra that would be expected for Van Assche's initial estimates of the Noddacks' residue compositions. The first results were surprisingly close to their published spectrum! Over the next couple of years, we refined our reconstruction of their analytical methods and performed more sophisticated simulations. The agreement between simulated and reported spectra improved further. Our calculation of the amount of element 43 required to produce their spectrum is quite similar to the direct measurements of natural technetium abundance in uranium ore published in 1999 by Dave Curtis and colleagues at Los Alamos. We can find no other plausible explanation for the Noddacks' data than that they did indeed detect fission "masurium."<br /> {{cite journal|last=Armstrong|first=J. T.|url=http://pubs.acs.org/cen/80th/technetium.html | doi = 10.1021/cen-v081n036.p110|title=Technetium|journal=Chemical & Engineering News|date=2003|volume=81|issue=36|pages=110}}</ref> |
German chemists ], ], and ] reported the discovery of element 75 and element 43 in 1925, and named element 43 '']'' (after ] in eastern ], now in ], the region where Walter Noddack's family originated).<ref name="multidict"/> The group bombarded ] with a beam of ]s and deduced element 43 was present by examining ] diffraction ]s.<ref name="Emsley2001p423">{{harvnb|Emsley|2001|p=423}}</ref> The ] of the X-rays produced is related to the atomic number by a formula derived by ] in 1913. The team claimed to detect a faint X-ray signal at a wavelength produced by element 43. Later experimenters could not replicate the discovery, and it was dismissed as an error for many years.<ref name="armstrong">{{cite journal|last=Armstrong |first=J. T.|url=http://pubs.acs.org/cen/80th/technetium.html | doi = 10.1021/cen-v081n036.p110|title=Technetium|publisher=Chemical & Engineering News|date=2003|accessdate = 2009-11-11}}</ref><ref>{{cite news| first = K. A.|last = Nies|url = http://www.hypatiamaze.org/ida/tacke.html|title = Ida Tacke and the warfare behind the discovery of fission|date = 2001| accessdate = 2009-05-05}}</ref> Still, in 1933, a series of articles on the discovery of elements quoted the name ''masurium'' for element 43.<ref>{{cite journal|title = The discovery of the elements. XX. Recently discovered elements|last = Weeks|first = M. E.|journal = Journal of Chemical Education|date = 1933|pages = 161–170|doi = 10.1021/ed010p161|volume = 10|issue = 3|bibcode = 1933JChEd..10..161W }}</ref><ref group=note>In 1998 John T. Armstrong of the ] ran "computer simulations" of the 1925 experiments and obtained results quite close to those reported by the Noddack team. "Using first-principles X-ray-emission spectral-generation algorithms developed at NIST, I simulated the X-ray spectra that would be expected for Van Assche's initial estimates of the Noddacks' residue compositions. The first results were surprisingly close to their published spectrum! Over the next couple of years, we refined our reconstruction of their analytical methods and performed more sophisticated simulations. The agreement between simulated and reported spectra improved further. Our calculation of the amount of element 43 required to produce their spectrum is quite similar to the direct measurements of natural technetium abundance in uranium ore published in 1999 by Dave Curtis and colleagues at Los Alamos. We can find no other plausible explanation for the Noddacks' data than that they did indeed detect fission "masurium."<br /> {{cite journal|last=Armstrong|first=J. T.|url=http://pubs.acs.org/cen/80th/technetium.html | doi = 10.1021/cen-v081n036.p110|title=Technetium|journal=Chemical & Engineering News|date=2003|volume=81|issue=36|pages=110}}</ref> Whether the 1925 team actually did discover element 43 is still debated.<ref>{{cite journal|title = From Masurium to Trinacrium: The Troubled Story of Element 43|journal = Journal of Chemical Education|date = 2005|volume = 82|pages = 221–227|first = R.|last = Zingales|url = http://jchemed.chem.wisc.edu/HS/Journal/Issues/2005/Feb/abs221.html|doi = 10.1021/ed082p221|bibcode = 2005JChEd..82..221Z|issue = 2 }}</ref> | ||
| ===Official discovery and later history=== | ===Official discovery and later history=== | ||
| The ] of element 43 was finally confirmed in a December 1936 experiment at the ] in Sicily |
The ] of element 43 was finally confirmed in a December 1936 experiment at the ] in Sicily by ] and ].<ref name="Heiserman1992p164">{{harvnb| Heiserman|1992|p=164}}</ref> In mid-1936, Segrè visited the United States, first ] in New York and then the ] in California. He persuaded ] inventor ] to let him take back some discarded cyclotron parts that had become ]. Lawrence mailed him a molybdenum foil that had been part of the deflector in the cyclotron.<ref>{{cite book |first=Emilio |last=Segrè |date=1993 |title=A Mind Always in Motion: the Autobiography of Emilio Segrè |publisher=University of California Press |location=Berkeley, California |isbn=0520076273 |pages=115–118}}</ref> | ||
| Segrè enlisted his colleague Perrier to attempt to prove, through comparative chemistry, that the molybdenum activity was indeed from an element with the atomic number 43. They succeeded in isolating the ]s ]m and ].<ref name=segre/><ref name=blocks>{{cite book| title = Nature's Building Blocks: An A-Z Guide to the Elements|last = Emsley |first=J. |location = New York| publisher = Oxford University Press| date = 2001| isbn = 0-19-850340-7| pages = 422–425|url=https://books.google.com/?id=Yhi5X7OwuGkC&pg=PA423}}</ref> University of Palermo officials wanted them to name their discovery "''panormium''", after the ] name for ], ''Panormus''. In 1947<ref name=segre>{{cite journal|doi = 10.1038/159024a0|pmid = 20279068|title = Technetium: The Element of Atomic Number 43|date = 1947|last1 = Perrier|first1 = C.|last2 = Segrè|first2 = E.|journal = Nature|volume = 159|issue = 4027|pages = 24|bibcode = 1947Natur.159...24P }}</ref> element 43 was named after the ] word ''τεχνητός'', meaning "artificial", since it was the first element to be artificially produced.<ref name="history-origin"/><ref name="multidict">{{cite news| title = Elentymolgy and Elements Multidict, "Technetium"| url = http://elements.vanderkrogt.net/element.php?sym=Tc| accessdate = 2009-05-05| last = van der Krogt |first=P.}}</ref> Segrè returned to Berkeley and met ]. They isolated the ] ], which is now used in some ten million medical diagnostic procedures annually.<ref>{{cite book |title = The transuranium people: The inside story |publisher =University of California, Berkeley & Lawrence Berkeley National Laboratory|date =2000 |chapter =Chapter 1.2: Early Days at the Berkeley Radiation Laboratory|page =15|url =http://www.worldscibooks.com/physics/p074.html|isbn =1-86094-087-0}}</ref> | Segrè enlisted his colleague Perrier to attempt to prove, through comparative chemistry, that the molybdenum activity was indeed from an element with the atomic number 43. They succeeded in isolating the ]s ]m and ].<ref name=segre/><ref name=blocks>{{cite book| title = Nature's Building Blocks: An A-Z Guide to the Elements|last = Emsley |first=J. |location = New York| publisher = Oxford University Press| date = 2001| isbn = 0-19-850340-7| pages = 422–425|url=https://books.google.com/?id=Yhi5X7OwuGkC&pg=PA423}}</ref> University of Palermo officials wanted them to name their discovery "''panormium''", after the ] name for ], ''Panormus''. In 1947<ref name=segre>{{cite journal|doi = 10.1038/159024a0|pmid = 20279068|title = Technetium: The Element of Atomic Number 43|date = 1947|last1 = Perrier|first1 = C.|last2 = Segrè|first2 = E.|journal = Nature|volume = 159|issue = 4027|pages = 24|bibcode = 1947Natur.159...24P }}</ref> element 43 was named after the ] word ''τεχνητός'', meaning "artificial", since it was the first element to be artificially produced.<ref name="history-origin"/><ref name="multidict">{{cite news| title = Elentymolgy and Elements Multidict, "Technetium"| url = http://elements.vanderkrogt.net/element.php?sym=Tc| accessdate = 2009-05-05| last = van der Krogt |first=P.}}</ref> Segrè returned to Berkeley and met ]. They isolated the ] ], which is now used in some ten million medical diagnostic procedures annually.<ref>{{cite book |title = The transuranium people: The inside story |publisher =University of California, Berkeley & Lawrence Berkeley National Laboratory|date =2000 |chapter =Chapter 1.2: Early Days at the Berkeley Radiation Laboratory|page =15|url =http://www.worldscibooks.com/physics/p074.html|isbn =1-86094-087-0}}</ref> | ||
| In 1952, astronomer ] in California detected the ] of technetium (specifically ]s of 403.1 ], 423.8 nm, 426.2 nm, and 429.7 nm) in light from ] ]s.<ref>{{cite journal|last=Merrill |first=P. W.|journal=Science|volume=115|pages=479–89 |date=1952|title=Technetium in the stars|doi=10.1126/science.115.2992.479|issue=2992|bibcode = 1952Sci...115..479. }}</ref> The stars were near the end of their lives, yet were rich in this short-lived element, |
In 1952, astronomer ] in California detected the ] of technetium (specifically ]s of 403.1 ], 423.8 nm, 426.2 nm, and 429.7 nm) in light from ] ]s.<ref>{{cite journal|last=Merrill |first=P. W.|journal=Science|volume=115|pages=479–89 |date=1952|title=Technetium in the stars|doi=10.1126/science.115.2992.479|issue=2992|bibcode = 1952Sci...115..479. }}</ref> The stars were near the end of their lives, yet were rich in this short-lived element, indicating that it was being produced in the starts by ]s. This evidence bolstered the hypothesis that heavier elements are the product of ] in stars.<ref name=blocks/> More recently, such observations provided evidence that elements are formed by ] in the ].<ref name=s8>{{harvnb|Schwochau|2000|pp=7–9}}</ref> | ||
| Since |
Since that discovery, there have been many searches in terrestrial materials for natural sources of technetium. In 1962, technetium-99 was isolated and identified in ] from the ] in extremely small quantities (about 0.2 ng/kg);<ref name=s8/> there it originates as a ] product of ]. The ] ] contains evidence that significant amounts of technetium-99 were produced and have since decayed into ].<ref name=s8/> | ||
| ==Characteristics== | ==Characteristics== | ||
| ===Physical properties=== | ===Physical properties=== | ||
| Technetium is a silvery-gray radioactive ] with an appearance similar to |
Technetium is a silvery-gray radioactive ] with an appearance similar to ], commonly obtained as a gray powder.<ref name=CRC/> The ] of the pure metal is ] ]. Atomic technetium has characteristic ] at these ]s of light: 363.3 ], 403.1 nm, 426.2 nm, 429.7 nm, and 485.3 nm.<ref>{{cite book| title = The CRC Handbook| publisher =CRC press|chapter = Line Spectra of the Elements| date = 2004–2005|url=https://books.google.com/?id=q2qJId5TKOkC&pg=PT1672|pages=10–70 (1672) | first=David R. | last=Lide | isbn=978-0-8493-0595-5}}</ref> | ||
| The metal form is slightly ], meaning its ] align with external ]s, but will assume random orientations once the field is removed.<ref name=enc/> Pure, metallic, single-crystal technetium becomes a ] at temperatures below 7.46 ].<ref group=note>Irregular crystals and trace impurities raise this transition temperature to 11.2 K for 99.9% pure technetium powder.{{harv|Schwochau|2000|p=96}}</ref><ref>{{harvnb|Schwochau|2000|p=96}}</ref> Below this temperature, technetium has a very high ], |
The metal form is slightly ], meaning its ] align with external ]s, but will assume random orientations once the field is removed.<ref name=enc/> Pure, metallic, single-crystal technetium becomes a ] at temperatures below 7.46 ].<ref group=note>Irregular crystals and trace impurities raise this transition temperature to 11.2 K for 99.9% pure technetium powder.{{harv|Schwochau|2000|p=96}}</ref><ref>{{harvnb|Schwochau|2000|p=96}}</ref> Below this temperature, technetium has a very high ], greater than any other element except ].<ref>{{cite news| title = Technetium as a Material for AC Superconductivity Applications| last = Autler |first=S. H.| publisher = Proceedings of the 1968 Summer Study on Superconducting Devices and Accelerators|accessdate = 2009-05-05|date=1968| url = http://www.bnl.gov/magnets/Staff/Gupta/Summer1968/0049.pdf}}</ref> | ||
| ===Chemical properties=== | ===Chemical properties=== | ||
| Technetium is |
Technetium is located in the ] of the periodic table, between ] and ]. As predicted by the ], its chemical properties are between those two elements. Of the two, technetium more closely resembles rhenium, particularly in its chemical inertness and tendency to form ]s.<ref>{{harvnb|Greenwood|1997|p=1044}}</ref> Unlike manganese, technetium does not readily form ]s (]s with a net positive charge). Technetium exhibits nine ]<nowiki/>s from −1 to +7, with +4, +5, and +7 being the most common.<ref name="LANL"/> Technetium dissolves in ], ], and concentrated ], but it is not soluble in ] of any concentration.<ref name= CRC/> | ||
| Technetium can catalyse the destruction of ] by ], and this property is to its multiplicity of valencies.<ref>{{cite journal | doi = 10.1016/0022-5088(84)90023-7 | title=The technetium-catalysed oxidation of hydrazine by nitric acid | journal=Journal of the Less Common Metals | date=1984 | volume=97 | pages=191–203 | first=John | last=Garraway}}</ref> This caused a problem in the separation of plutonium from uranium in ], where hydrazine is used as a protective reductant to keep plutonium in the trivalent rather than the more stable tetravalent state. The problem was exacerbated by the mutually-enhanced solvent extraction of technetium and zirconium at the previous stage,<ref>{{cite journal | doi = 10.1016/0022-5088(85)90379-0 | title=Coextraction of pertechnetate and zirconium by tri-n-butyl phosphate | journal=Journal of the Less Common Metals | date=1985 | volume=106 | issue=1 | pages=183–192 | first=J. | last=Garraway}}</ref> and required a process modification. | |||
| ====Hydride and oxides==== | ====Hydride and oxides==== | ||
| The reaction of technetium with ] produces the negatively charged ] {{chem|TcH|9|2-}} ion, which has the same type of ] as (is isostructural with) ]. It consists of a trigonal prism with a technetium atom in the center and six ]s at the corners. Three more hydrogen atoms make a triangle lying parallel to the base and crossing the prism in its center. Although those hydrogen atoms are not equivalent geometrically, their electronic structure is almost the same. This complex has a ] of 9 (meaning that the technetium atom has nine neighbors), which is the highest for a technetium complex. Two hydrogen atoms in the complex can be replaced by sodium (Na<sup>+</sup>) or potassium (K<sup>+</sup>) ions.<ref>{{harvnb|Schwochau|2000|p=146}}</ref> | The reaction of technetium with ] produces the negatively charged ] {{chem|TcH|9|2-}} ion, which has the same type of ] as (in other words, it is ] with) ]. It consists of a trigonal prism with a technetium atom in the center and six ]s at the corners. Three more hydrogen atoms make a triangle lying parallel to the base and crossing the prism in its center. Although those hydrogen atoms are not equivalent geometrically, their electronic structure is almost the same. This complex has a ] of 9 (meaning that the technetium atom has nine neighbors), which is the highest for a technetium complex. Two hydrogen atoms in the complex can be replaced by sodium (Na<sup>+</sup>) or potassium (K<sup>+</sup>) ions.<ref>{{harvnb|Schwochau|2000|p=146}}</ref> | ||
| ]|alt=Skeletal formula of technetium hydride described in the text.]] | ]|alt=Skeletal formula of technetium hydride described in the text.]] | ||
| Line 90: | Line 90: | ||
| Black-colored technetium dioxide (TcO<sub>2</sub>) can be produced by reduction of heptoxide with technetium or hydrogen.<ref>{{harvnb|Schwochau|2000|p=108}}</ref> | Black-colored technetium dioxide (TcO<sub>2</sub>) can be produced by reduction of heptoxide with technetium or hydrogen.<ref>{{harvnb|Schwochau|2000|p=108}}</ref> | ||
| ] (HTcO<sub>4</sub>) is produced by reacting Tc<sub>2</sub>O<sub>7</sub> with water or ]s, such as ], concentrated sulfuric acid, ], or a mixture of nitric and hydrochloric acids.<ref>{{harvnb|Schwochau|2000|p=127}}</ref> The resulting dark red, ] substance is a strong acid and easily donates protons. In concentrated ] Tc(VII) tetraoxidotechnetate anion converts to the octahedral form of technetic(VII) acid TcO<sub>3</sub>(OH)(H<sub>2</sub>O)<sub>2</sub>.<ref>{{cite journal|display-authors=7|author=Poineau F|author2=Weck PF|author3=German K|author4=Maruk A|author5=Kirakosyan G|author6= Lukens W|author7=Rego DB|author8=Sattelberger AP|author9=Czerwinski KR|title= Speciation of heptavalent technetium in sulfuric acid: structural and spectroscopic studies|journal= Dalton Transactions|date= 2010|volume= 39 |issue=37|pages=8616–8619|doi=10.1039/C0DT00695E |url=http://radchem.nevada.edu/docs/pub/tc%20in%20h2so4%20%28dalton%29%202010-08-23.pdf|pmid=20730190}}</ref> | ] (HTcO<sub>4</sub>) is produced by reacting Tc<sub>2</sub>O<sub>7</sub> with water or ]s, such as ], concentrated sulfuric acid, ], or a mixture of nitric and hydrochloric acids.<ref>{{harvnb|Schwochau|2000|p=127}}</ref> The resulting dark red, ] substance is a strong acid and easily donates protons. In concentrated ], Tc(VII) tetraoxidotechnetate anion converts to the octahedral form of technetic(VII) acid TcO<sub>3</sub>(OH)(H<sub>2</sub>O)<sub>2</sub>.<ref>{{cite journal|display-authors=7|author=Poineau F|author2=Weck PF|author3=German K|author4=Maruk A|author5=Kirakosyan G|author6= Lukens W|author7=Rego DB|author8=Sattelberger AP|author9=Czerwinski KR|title= Speciation of heptavalent technetium in sulfuric acid: structural and spectroscopic studies|journal= Dalton Transactions|date= 2010|volume= 39 |issue=37|pages=8616–8619|doi=10.1039/C0DT00695E |url=http://radchem.nevada.edu/docs/pub/tc%20in%20h2so4%20%28dalton%29%202010-08-23.pdf|pmid=20730190}}</ref> | ||
| The ] (tetroxidotechnetate) anion {{chem|TcO|4|-}} consists of a ] with oxygens in the corners and a technetium atom in the center. Unlike ] ({{chem|MnO|4|-}}), it is only a weak oxidizing agent. |
The ] (tetroxidotechnetate) anion {{chem|TcO|4|-}} consists of a ] with oxygens in the corners and a technetium atom in the center. Unlike ] ({{chem|MnO|4|-}}), it is only a weak oxidizing agent. ] is often used as a convenient water-soluble source of technetium isotopes, such as <sup>99m</sup>Tc, and as a ].<ref>{{harvnb|Schwochau|2000|pp=127–136}}</ref> | ||
| ====Sulfides, selenides, and tellurides==== | ====Sulfides, selenides, and tellurides==== | ||
| Technetium forms various ]s. TcS<sub>2</sub> is obtained by direct |
Technetium forms various ]s. TcS<sub>2</sub> is obtained by direct reacting technetium with elemental ], while Tc<sub>2</sub>S<sub>7</sub> is formed from ] as follows: | ||
| : 2 HTcO<sub>4</sub> + 7 H<sub>2</sub>S → Tc<sub>2</sub>S<sub>7</sub> + 8 H<sub>2</sub>O | : 2 HTcO<sub>4</sub> + 7 H<sub>2</sub>S → Tc<sub>2</sub>S<sub>7</sub> + 8 H<sub>2</sub>O | ||
| In this reaction technetium is ] to Tc(IV) while excess sulfur forms a disulfide ligand. The produced technetium heptasulfide has a polymeric structure (Tc<sub>3</sub>(µ<sup>3</sup>–S)(S<sub>2</sub>)<sub>3</sub>S<sub>6</sub>)<sub>''n''</sub> with a core similar to Mo<sub>3</sub>(µ<sup>3</sup>–S)(S<sub>2</sub>)<sub>6</sub><sup>2−</sup>.<ref>{{cite journal|author=Lukens, W. W.|first2=Jerome J. |last2=Bucher|title=Evolution of Technetium Speciation in Reducing Grout Waste Forms|pages=8064–8070|date=2005|url=http://pbadupws.nrc.gov/docs/ML0527/ML052720213.pdf|pmid=16295876|doi=10.1021/es050155c|journal=Environmental Science & Technology|volume=39|issue=20|last3=Shuh|first3=David K.|last4=Edelstein|first4=Norman M.|bibcode = 2005EnST...39.8064L }}</ref> | In this reaction, technetium is ] to Tc(IV) while excess sulfur forms a disulfide ligand. The produced technetium heptasulfide has a polymeric structure (Tc<sub>3</sub>(µ<sup>3</sup>–S)(S<sub>2</sub>)<sub>3</sub>S<sub>6</sub>)<sub>''n''</sub> with a core similar to Mo<sub>3</sub>(µ<sup>3</sup>–S)(S<sub>2</sub>)<sub>6</sub><sup>2−</sup>.<ref>{{cite journal|author=Lukens, W. W.|first2=Jerome J. |last2=Bucher|title=Evolution of Technetium Speciation in Reducing Grout Waste Forms|pages=8064–8070|date=2005|url=http://pbadupws.nrc.gov/docs/ML0527/ML052720213.pdf|pmid=16295876|doi=10.1021/es050155c|journal=Environmental Science & Technology|volume=39|issue=20|last3=Shuh|first3=David K.|last4=Edelstein|first4=Norman M.|bibcode = 2005EnST...39.8064L }}</ref> | ||
| Upon heating, technetium heptasulfide decomposes into disulfide and elementary sulfur: | Upon heating, technetium heptasulfide decomposes into disulfide and elementary sulfur: | ||
| Line 111: | Line 111: | ||
| ; {{harvnb|Schwochau|2000|p=176}}</ref>|alt=Skeletal formula featuring a technetium atom in its center, symmetrically bonded to four nitrogen atoms in a plane and to one oxygen atom perpendicular to the plane. Nitrogen atoms are terminated by OH, C-CH<sub>3</sub> and C-C-CH<sub>3</sub> groups.]] | ; {{harvnb|Schwochau|2000|p=176}}</ref>|alt=Skeletal formula featuring a technetium atom in its center, symmetrically bonded to four nitrogen atoms in a plane and to one oxygen atom perpendicular to the plane. Nitrogen atoms are terminated by OH, C-CH<sub>3</sub> and C-C-CH<sub>3</sub> groups.]] | ||
| Technetium forms numerous organic complexes, |
Technetium forms numerous organic complexes, relatively well-investigated because they are important for nuclear medicine. Technetium carbonyl (Tc<sub>2</sub>(CO)<sub>10</sub>) is a white solid.<ref>{{cite journal|doi = 10.1021/ja01474a038|date = 1961|last1 = Hileman|first1 = J. C.|last2 = Huggins|last3 = Kaesz|journal = Journal of the American Chemical Society|volume = 83|title = Technetium carbonyl|pages = 2953–2954|first2 = D. K.|first3 = H. D.|issue = 13}}</ref> In this molecule, two technetium atoms are weakly bound to each other; each atom is surrounded by ] of five carbonyl ligands. The bond length between technetium atoms, 303 pm,<ref>{{cite journal|title = The Crystal Structure of Ditechnetium Decacarbonyl|doi =10.1021/ic50030a011|date =1965|last1 =Bailey|first1 =M. F.|journal =Inorganic Chemistry|volume =4|pages =1140–1145|last2 = Dahl|first2 = Lawrence F.|issue = 8}}</ref><ref>{{cite journal|doi = 10.1107/S0365110X62002789|title = Unit cell and space group of technetium carbonyl, Tc2(CO)10|date = 1962|last1 = Wallach|first1 = D.|journal = Acta Crystallographica|volume = 15|page = 1058|issue = 10}}</ref> is significantly larger than the distance between two atoms in metallic technetium (272 pm). Similar ]s are formed by technetium's ], manganese and rhenium.<ref>{{harvnb|Schwochau|2000|pp=286, 328}}</ref> | ||
| A technetium complex<ref group=note>3,3,9,9-tetramethyl-4,8-diazaundecane-2,10-dione dioxime Hexamethyipropyleneamine Oxime (HMPAO)</ref> with an organic ligand (shown in the figure on right) is commonly used in nuclear medicine. It has a unique Tc−O ] (moiety) oriented perpendicularly to the plane of the molecule, where the oxygen atom can be replaced by a nitrogen atom.<ref>{{cite journal|doi = 10.1021/ic00224a031|title = Synthesis, characterization, and x-ray structural determinations of technetium(V)-oxo-tetradentate amine oxime complexes|date = 1986|last1 = Jurisson|first1 = S.|journal = Inorganic Chemistry|volume = 25|pages = 543–549|last2 = Schlemper|first2 = E. O.|last3 = Troutner|first3 = D. E.|last4 = Canning|first4 = L. R.|last5 = Nowotnik|first5 = D. P.|last6 = Neirinckx|first6 = R. D.|issue = 4}}</ref> | A technetium complex<ref group=note>3,3,9,9-tetramethyl-4,8-diazaundecane-2,10-dione dioxime Hexamethyipropyleneamine Oxime (HMPAO)</ref> with an organic ligand (shown in the figure on right) is commonly used in nuclear medicine. It has a unique Tc−O ] (moiety) oriented perpendicularly to the plane of the molecule, where the oxygen atom can be replaced by a nitrogen atom.<ref>{{cite journal|doi = 10.1021/ic00224a031|title = Synthesis, characterization, and x-ray structural determinations of technetium(V)-oxo-tetradentate amine oxime complexes|date = 1986|last1 = Jurisson|first1 = S.|journal = Inorganic Chemistry|volume = 25|pages = 543–549|last2 = Schlemper|first2 = E. O.|last3 = Troutner|first3 = D. E.|last4 = Canning|first4 = L. R.|last5 = Nowotnik|first5 = D. P.|last6 = Neirinckx|first6 = R. D.|issue = 4}}</ref> | ||
| Line 117: | Line 117: | ||
| ===Isotopes=== | ===Isotopes=== | ||
| {{main article|Isotopes of technetium}} | {{main article|Isotopes of technetium}} | ||
| Technetium, with ] (denoted ''Z'') 43, is the lowest-numbered element in the periodic table |
Technetium, with ] (denoted ''Z'') 43, is the lowest-numbered element in the periodic table of which all isotopes are ]. The second-lightest, exclusively radioactive element, ], has an atomic number of 61.<ref name="LANL"/> ] with an odd number of ]s are less stable than those with even numbers, even when the total number of ]s (protons + ]s) is even,<ref>{{cite book|url=https://books.google.com/?id=8HSGFThnbvkC&pg=PA547|page=547|title=Principles of stellar evolution and nucleosynthesis: with a new preface|author=Clayton, D. D.|publisher=University of Chicago Press|date=1983|isbn=0-226-10953-4}}</ref> and odd numbered elements have fewer stable ]s. | ||
| The most stable ] are technetium-98 with a ] of 4.2 million years (]), technetium-97 |
The most stable ] are technetium-98 with a ] of 4.2 million years (]), technetium-97 with 2.6 Ma, and technetium-99 with 211,000 years.<ref name="NNDC"/> Thirty other radioisotopes have been characterized with ]s ranging from 85 to 118.<ref name="NNDC">{{cite web | ||
| |url = http://www.nndc.bnl.gov/chart/ | |url = http://www.nndc.bnl.gov/chart/ | ||
| |author = NNDC contributors | |author = NNDC contributors | ||
| Line 126: | Line 126: | ||
| |accessdate = 2009-11-11 | |accessdate = 2009-11-11 | ||
| |date = 2008 | |date = 2008 | ||
| |location = New York}}</ref> Most of these have half-lives that are less than an hour |
|location = New York}}</ref> Most of these have half-lives that are less than an hour, the exceptions being technetium-93 (half-life: 2.73 hours), technetium-94 (half-life: 4.88 hours), technetium-95 (half-life: 20 hours), and technetium-96 (half-life: 4.3 days).<ref name="CRCisotopes"/> | ||
| The primary ] for isotopes lighter than technetium-98 (<sup>98</sup>Tc) is ], |
The primary ] for isotopes lighter than technetium-98 (<sup>98</sup>Tc) is ], producing ] (''Z'' = 42).<ref name="NNDC"/> For technetium-98 and heavier isotopes, the primary mode is ] (the emission of an ] or ]), producing ] (''Z'' = 44), with the exception that technetium-100 can decay both by beta emission and electron capture.<ref name="NNDC"/><ref>{{cite book| title = The CRC Handbook of Chemistry and Physics| publisher =CRC press| chapter = Table of the isotopes| date = 2004–2005 | editor = Lide, David R.}}</ref> | ||
| Technetium also has numerous ]s, which are isotopes with one or more ] nucleons. Technetium-97m (<sup>97m</sup>Tc; 'm' stands for ]) is the most stable, with a half-life of 91 days (0.0965 MeV).<ref name="CRCisotopes">{{cite book | Technetium also has numerous ]s, which are isotopes with one or more ] nucleons. Technetium-97m (<sup>97m</sup>Tc; 'm' stands for ]) is the most stable, with a half-life of 91 days (0.0965 MeV).<ref name="CRCisotopes">{{cite book | ||
| Line 140: | Line 140: | ||
| |location = Boca Raton, Florida | |location = Boca Raton, Florida | ||
| |pages = 11–88–11–89 | |pages = 11–88–11–89 | ||
| |isbn = 0-8493-0487-3}}</ref> This is followed by technetium-95m (half-life: 61 days, 0.03 MeV), and technetium-99m (half-life: 6.01 hours, 0.142 MeV).<ref name="CRCisotopes"/> Technetium-99m |
|isbn = 0-8493-0487-3}}</ref> This is followed by technetium-95m (half-life: 61 days, 0.03 MeV), and technetium-99m (half-life: 6.01 hours, 0.142 MeV).<ref name="CRCisotopes"/> Technetium-99m emits only ]s and decays to technetium-99.<ref name="CRCisotopes"/> | ||
| Technetium-99 (<sup>99</sup>Tc) is a major product of the fission of uranium-235 (<sup>235</sup>U), making it the most common and most readily available isotope of technetium. One gram of technetium-99 produces 6.2×10<sup>8</sup> disintegrations a second (that is, 0.62 G]/g).<ref name=enc/> | Technetium-99 (<sup>99</sup>Tc) is a major product of the fission of uranium-235 (<sup>235</sup>U), making it the most common and most readily available isotope of technetium. One gram of technetium-99 produces 6.2×10<sup>8</sup> disintegrations a second (that is, 0.62 G]/g).<ref name=enc/> | ||
| Line 146: | Line 146: | ||
| ==Occurrence and production== | ==Occurrence and production== | ||
| ] | ] | ||
| Only minute traces of technetium occur naturally in the Earth's ]. This is because technetium-98's ] is only 4.2 million years. |
Only minute traces of technetium occur naturally in the Earth's ]. This is because technetium-98's ] is only 4.2 million years. More a thousand of such periods have passed since the formation of the ], so the probability for the survival of even one atom of ] technetium is effectively zero. However, small amounts exist as spontaneous ]s in ]s. A kilogram of uranium contains an estimated 1 nanogram (10<sup>−9</sup> g) of technetium.<ref name=blocks/><ref>{{cite journal|doi = 10.1021/ac961159q|title = Analysis of Naturally Produced Technetium and Plutonium in Geologic Materials|date = 1997|last1 = Dixon|first1 = P.|journal = Analytical Chemistry|volume = 69|pages = 1692–9|last2 = Curtis|first2 = David B.|last3 = Musgrave|first3 = John|last4 = Roensch|first4 = Fred|last5 = Roach|first5 = Jeff|last6 = Rokop|first6 = Don|issue = 9|pmid = 21639292}}</ref><ref>{{cite journal|doi =10.1016/S0016-7037(98)00282-8|title =Nature's uncommon elements: plutonium and technetium|first4 =Jan|last4 =Cramer|first3 =Paul|last3 =Dixon|first2 =June|date=1999|last2 =Fabryka-Martin|last1=Curtis|first1=D.|journal=Geochimica et Cosmochimica Acta|volume =63|pages =275|bibcode=1999GeCoA..63..275C|issue =2}}</ref> Some ] stars with the spectral types S-, M-, and N contain a spectral absorption line indicating the presence of technetium.<ref name=CRC>{{cite book| first= C. R.|last = Hammond |chapter=The Elements |title = Handbook of Chemistry and Physics |edition=81st| publisher =CRC press|isbn = 0-8493-0485-7| date= 2004}}</ref><ref>{{cite journal|doi = 10.1126/science.114.2951.59|pmid = 17782983|date = 1951|last1 = Moore|first1 = C.E.|title = Technetium in the Sun|volume = 114|issue = 2951|pages = 59–61|journal = Science |location=New York, N.Y.|bibcode=1951Sci...114...59M}}</ref><!--Technetium in Red Giant Stars P Merrill — Science, 1952--> These red-giants are known informally as ]s. | ||
| ===Fission waste product=== | ===Fission waste product=== | ||
| In contrast |
In contrast to the rare natural occurrence, bulk quantities of technetium-99 are produced each year from ], which contain various fission products. The fission of a gram of ] in ]s yields 27 mg of technetium-99, giving technetium a ] of 6.1%.<ref name=enc/> Other ] isotopes produce similar yields of technetium, such as 4.9% from ] and 6.21% from ].<ref>{{harvnb|Schwochau|2000|pp=374–404}}</ref> An estimated 49,000 T] (78 ]) of technetium was produced in nuclear reactors between 1983 and 1994, by far the dominant source of terrestrial technetium.<ref name="yoshihara">{{cite book| first = K.|last = Yoshihara| chapter = Technetium in the Environment| title = Topics in Current Chemistry: Technetium and Rhenium| volume = 176|editor = K. Yoshihara|editor2 = T. Omori|publisher = Springer-Verlag| location = Berlin Heidelberg|date = 1996|isbn=978-3-540-59469-7|doi=10.1007/3-540-59469-8_2|pages=17–35}}</ref><ref name=leon/> Only a fraction of the production is used commercially.<ref group=note>{{As of|2005}}, technetium-99 in the form of ] is available to holders of an ] permit:{{cite book| first= C. R.|last = Hammond |chapter=The Elements|title = Handbook of Chemistry and Physics |edition=81st |publisher =CRC press|isbn = 0-8493-0485-7| date= 2004}}</ref> | ||
| Technetium-99 is produced by the ] of both uranium-235 and plutonium-239. It is therefore present in ] and in the ] of ] explosions. Its decay, measured in becquerels per amount of spent fuel, is dominant after about 10<sup>4</sup> to 10<sup>6</sup> years after the creation of the nuclear waste.<ref name="yoshihara"/> From 1945 to 1994, an estimated 160 T] (about 250 kg) of technetium-99 was released into the environment |
Technetium-99 is produced by the ] of both uranium-235 and plutonium-239. It is therefore present in ] and in the ] of ] explosions. Its decay, measured in ] per amount of spent fuel, is dominant after about 10<sup>4</sup> to 10<sup>6</sup> years after the creation of the nuclear waste.<ref name="yoshihara"/> {{Clarification needed|reason="its decay ... is dominant"?? what does this mean?}} From 1945 to 1994, an estimated 160 T] (about 250 kg) of technetium-99 was released into the environment during atmospheric ]s.<ref name="yoshihara"/><ref>{{cite book|url=https://books.google.com/?id=QLHr-UYWoo8C&pg=PA69|page=69|title=Technetium in the environment|last1=Desmet |first1=G. |last2=Myttenaere |first2=C.|publisher=Springer|date=1986|isbn=0-85334-421-3}}</ref> The amount of technetium-99 from nuclear reactors released into the environment up to 1986 is on the order of 1000 TBq (about 1600 kg), primarily by ]; most of this was discharged into the sea. Reprocessing methods have reduced emissions since then, but as of 2005 the primary release of technetium-99 into the environment is by the ] plant, which released an estimated 550 TBq (about 900 kg) from 1995–1999 into the ].<ref name=leon>{{cite journal|url=http://www.radiochem.org/paper/JN63/jn6326.pdf|journal=Journal of Nuclear and Radiochemical Sciences|volume=6|issue=3|pages=253–259|date=2005|title=99Tc in the Environment: Sources, Distribution and Methods|last=Garcia-Leon |first=M.}}</ref> From 2000 onwards the amount has been limited by regulation to 90 TBq (about 140 kg) per year.<ref>{{cite journal|url = https://www.jstage.jst.go.jp/article/jnrs2000/4/1/4_1_A1/_article|title = Technetium-99 Behaviour in the Terrestrial Environment — Field Observations and Radiotracer Experiments|first = K.|last = Tagami|journal=Journal of Nuclear and Radiochemical Sciences|volume = 4|pages= A1–A8|date = 2003|doi=10.14494/jnrs2000.4.a1}}</ref> Discharge of technetium into the sea resulted in contamination of some seafood with minuscule quantities of this element. For example, ] and fish from west ] contain about 1 Bq/kg of technetium.<ref>{{cite book|url=https://books.google.com/?id=zVmdln2pJxUC&pg=PA403|page=403|title=Mineral components in foods|last1=Szefer |first1=P. |last2=Nriagu |first2=J. O.|publisher=CRC Press|date=2006|isbn=0-8493-2234-0}}</ref><ref>{{cite journal| title = Gut transfer and doses from environmental technetium|first1= J. D.|last1 = Harrison|first2 = A.|last2 = Phipps|date = 2001|journal = J. Radiol. Prot.|pages= 9–11| volume = 21 |doi = 10.1088/0952-4746/21/1/004| pmid = 11281541| issue = 1|bibcode = 2001JRP....21....9H }}</ref><ref group=note>The ], ]-forming ] in the '']'' ] are able to reduce Tc(VII) to Tc(IV). ''Clostridia'' bacteria play a role in reducing iron, ], and uranium, thereby affecting these elements' solubility in soil and sediments. Their ability to reduce technetium may determine a large part of mobility of technetium in industrial wastes and other subsurface environments. {{cite journal| last1=Francis |first1=A. J. |last2=Dodge |first2=C. J. |last3=Meinken |first3=G. E.|title = Biotransformation of pertechnetate by ''Clostridia'' |journal = Radiochimica Acta|volume = 90| date= 2002|pages = 791–797|doi= 10.1524/ract.2002.90.9-11_2002.791| issue=9–11}}</ref> | ||
| ===Fission product for commercial use=== | ===Fission product for commercial use=== | ||
| The ] isotope technetium-99m is continuously produced as a ] from the fission of uranium or ] in ]s. Because used fuel is allowed to stand for several years before reprocessing, all molybdenum-99 and technetium-99m |
The ] isotope technetium-99m is continuously produced as a ] from the fission of uranium or ] in ]s. Because used fuel is allowed to stand for several years before reprocessing, all molybdenum-99 and technetium-99m is decayed by the time that the fission products are separated from the major ]s in conventional ]. The liquid left after plutonium–uranium extraction (]) contains a high concentration of technetium as {{chem|TcO|4|-}} but almost all of this is technetium-99, not technetium-99m.<ref>{{harvnb|Schwochau|2000|p=39}}</ref> | ||
| The vast majority of the technetium-99m used in medical work is produced by irradiating dedicated ] targets in a reactor, extracting molybdenum-99 from the targets in reprocessing facilities,<ref>{{cite journal|last=Moore|first=P.W.|title=Technetium-99 in generator systems|journal=Journal of Nuclear Medicine |date=April 1984|volume=25 |issue=4|pages=499–502 |pmid=6100549|url=http://jnm.snmjournals.org/content/25/4/499.full.pdf|accessdate=2012-05-11}}</ref> and recovering at the diagnostic center the technetium-99m |
The vast majority of the technetium-99m used in medical work is produced by irradiating dedicated ] targets in a reactor, extracting molybdenum-99 from the targets in reprocessing facilities,<ref>{{cite journal|last=Moore|first=P.W.|title=Technetium-99 in generator systems|journal=Journal of Nuclear Medicine |date=April 1984|volume=25 |issue=4|pages=499–502 |pmid=6100549|url=http://jnm.snmjournals.org/content/25/4/499.full.pdf|accessdate=2012-05-11}}</ref> and recovering at the diagnostic center the technetium-99m produced upon decay of molybdenum-99.<ref>{{cite patent|country=US|number=3799883|title=Silver coated charcoal step|invent1=Hirofumi Arino|assign1= Union Carbide Corporation|gdate=March 26, 1974}}</ref><ref name="NAS Report">{{cite book| title = Medical Isotope Production Without Highly Enriched Uranium| url = http://www.nap.edu/catalog/12569.html| accessdate = 2009-08-27| author = Committee on Medical Isotope Production Without Highly Enriched Uranium| publisher = National Academies Press|page=vii|isbn=0-309-13040-9|date=2009}}</ref> Molybdenum-99 in the form of molybdate {{chem|MoO|4|2-}} is ] onto acid alumina ({{chem|Al|2|O|3}}) in a ] ] inside a ] ("technetium cow", also occasionally called a "molybdenum cow"). Molybdenum-99 has a half-life of 67 hours, so short-lived technetium-99m (half-life: 6 hours), which results from its decay, is being constantly produced.<ref name=blocks/> The soluble ] {{chem|TcO|4|-}} can then be chemically extracted by ] using a ]. A drawback of this process is that it requires targets containing uranium-235, which are subject to the security precautions of fissile materials.<ref>{{cite news|title=Nuclear forensics sleuths trace the origin of trafficked material|url=http://arq.lanl.gov/source/orgs/nmt/nmtdo/AQarchive/4thQuarter07/page1.shtml|publisher=Los Alamos National Laboratory|accessdate=2009-11-11|last=Lützenkirchen |first=K.-R.}}</ref><ref>{{cite news| author = Snelgrove, J. L.|first2=G. L.|last2=Hofman| url = http://www.rertr.anl.gov/MO99/JLS.pdf|title = Development and Processing of LEU Targets for Mo-99 Production|date = 1995| accessdate= 2009-05-05|work=ANL.gov, Presented at the 1995 International Meeting on Reduced Enrichment for Research and Test Reactors, September 18–21, 1994, Paris, France}}</ref> | ||
| A drawback of this process is that it requires targets containing uranium-235, which are subject to the security precautions of fissile materials.<ref>{{cite news|title=Nuclear forensics sleuths trace the origin of trafficked material|url=http://arq.lanl.gov/source/orgs/nmt/nmtdo/AQarchive/4thQuarter07/page1.shtml|publisher=Los Alamos National Laboratory|accessdate=2009-11-11|last=Lützenkirchen |first=K.-R.}}</ref><ref>{{cite news| author = Snelgrove, J. L.|first2=G. L.|last2=Hofman| url = http://www.rertr.anl.gov/MO99/JLS.pdf|title = Development and Processing of LEU Targets for Mo-99 Production|date = 1995| accessdate= 2009-05-05|work=ANL.gov, Presented at the 1995 International Meeting on Reduced Enrichment for Research and Test Reactors, September 18–21, 1994, Paris, France}}</ref> | |||
| Almost two-thirds of the world's supply comes from two reactors; the ] at ] in Ontario, Canada, and the ] at ] in Petten, Netherlands. All major technetium-99m |
Almost two-thirds of the world's supply comes from two reactors; the ] at ] in Ontario, Canada, and the ] at ] in Petten, Netherlands. All major reactors that produce technetium-99m were built in the 1960s and are close to the ]. The two new Canadian ] reactors planned and built to produce 200% of the demand of technetium-99m relieved all other producers from building their own reactors. With the cancellation of the already tested reactors in 2008, the future supply of technetium-99m became problematic.<ref>{{cite journal | last1 = Thomas | first1 = Gregory S. | last2 = Maddahi | first2 = Jamshid | title = The technetium shortage | journal = ] | volume = 17 | pages = 993–8 | date = 2010 | doi = 10.1007/s12350-010-9281-8 | issue = 6 | pmid=20717761}}</ref> | ||
| However the Chalk River reactor |
However, the Chalk River reactor was shut down for maintenance since August 2009, with an expected reopening in April 2010, and the Petten reactor had a 6-month scheduled maintenance shutdown beginning on Friday, February 19, 2010. With millions of procedures relying on technetium-99m every year, the low supply has left a gap, leaving some practitioners to revert to techniques not used for 20 years. Somewhat allaying this issue is an announcement from the Polish ] that they have developed a technique to isolate technetium.<ref name = "NY Times">{{cite news|url=http://www.nytimes.com/2010/02/17/health/17isotope.html?ref=science|publisher=New York Times|author=Wals, M. L.|date=February 16, 2010|title=New Source Of an Isotope In Medicine Is Found}}</ref> The reactor at Chalk River Laboratory reopened in August 2010 and the Petten reactor reopened September 2010.<ref>{{cite web| publisher = Clinical Oncology News | title = Medical Isotope Shortage Nearing End—For Now | first = Gina | last = Shaw | url = http://www.clinicaloncology.com/index.asp?section_id=150&show=dept&issue_id=674&article_id=16057 | date= October 2010 | accessdate = 2010-11-02}}</ref><!--http://www.nrg.eu/hfr-repair/ http://www.aecl.ca/NewsRoom/Community_Bulletins/100901.htm--> | ||
| ===Waste disposal=== | ===Waste disposal=== | ||
| The long half-life of technetium-99 and |
The long half-life of technetium-99 and property of forming an ] species creates a major concern for long-term disposal of radioactive waste. Many of the processes designed to remove fission products in reprocessing plants aim at ] species like ] (e.g., ]) and ] (e.g., ]). Hence the pertechnetate is able to escape through these treatment processes. Current disposal options favor ] in continental, geologically stable rock. The primary danger with such a course is that the waste is likely to come into contact with water, which could leach radioactive contamination into the environment. The anionic pertechnetate and ] do not adsorb well onto the surfaces of minerals, so they are likely to be washed away. By comparison ], ], and ] are much more able to bind to soil particles. Technetium could also be immobilized by some environments, such as lake bottom sediments, due to microbial activity;<ref>{{cite journal | url = http://www.springerlink.com/content/1066-3622/45/3/ | last1 = German | first1 = Konstantin E. | last2 = Firsova | first2 = E.V. | title = Bioaccumulation of Tc, Pu, and Np on Bottom Sediments in Two Types of Freshwater Lakes of the Moscow Oblast | journal = Radiochemistry | volume = 45 | pages = 250–6 | date = 2003 | issue = 6 | doi = 10.1023/A:1026008108860 | issn = 1608-3288 | last3 = Peretrukhin | first3 = V. F. | last4 = Khizhnyak | first4 = T. V. | last5 = Simonoff | first5 = M. }}</ref> | ||
| for this reason, the environmental chemistry of technetium is an active area of research.<ref>{{cite book|url=https://books.google.com/?id=eEeJbur_je0C&pg=PA147|page=147|title=Radioactivity in the terrestrial environment|last=Shaw |first=G. |publisher=Elsevier |date=2007 |isbn=0-08-043872-5}}</ref> | for this reason, the environmental chemistry of technetium is an active area of research.<ref>{{cite book|url=https://books.google.com/?id=eEeJbur_je0C&pg=PA147|page=147|title=Radioactivity in the terrestrial environment|last=Shaw |first=G. |publisher=Elsevier |date=2007 |isbn=0-08-043872-5}}</ref> | ||
Revision as of 00:50, 9 July 2016
Chemical element with atomic number 43 (Tc)Technetium (/tɛkˈniːʃiəm/) is a chemical element with symbol Tc and atomic number 43. It is the lightest element of which all isotopes are radioactive; none are stable. Only one other element, promethium, is followed (in the periodic table) by elements with stable isotopes. Nearly all technetium is produced synthetically, and only minute amounts are found in the Earth's crust. Naturally occurring technetium is a spontaneous fission product in uranium ore or the product of neutron capture in molybdenum ores. The chemical properties of this silvery gray, crystalline transition metal are intermediate between rhenium and manganese.
Many of technetium's properties were predicted by Dmitri Mendeleev before the element was discovered. Mendeleev noted a gap in his periodic table and gave the undiscovered element the provisional name ekamanganese (Em). In 1937, technetium (specifically the technetium-97 isotope) became the first predominantly artificial element to be produced, hence its name (from the Greek Error: {{Lang}}: text has italic markup (help), meaning "artificial", + -ium).
Its short-lived gamma ray-emitting nuclear isomer—technetium-99m—is used in nuclear medicine for a wide variety of diagnostic tests. Technetium-99 is used as a gamma-ray-free source of beta particles. Long-lived technetium isotopes produced commercially are by-products of fission of uranium-235 in nuclear reactors and are extracted from nuclear fuel rods. Because no isotope of technetium has a half-life longer than 4.2 million years (technetium-98), the 1952 detection of technetium in red giants, which are billions of years old, helped to prove that stars can produce heavier elements.
History
Search for element 43
From the 1860s through 1871, early forms of the periodic table proposed by Dmitri Mendeleev contained a gap between molybdenum (element 42) and ruthenium (element 44). In 1871, Mendeleev predicted this missing element would occupy the empty place below manganese and have similar chemical properties. Mendeleev gave it the provisional name ekamanganese (from eka-, the Sanskrit word for one) because the predicted element was one place down from the known element manganese.
Early mis-identifications
Many early researchers, both before and after the periodic table was published, were eager to be the first to discover and name the missing element; its location in the table suggested that it should be easier to find than other undiscovered elements.
| Year | Claimant | Suggested Name | Actual material |
|---|---|---|---|
| 1828 | Gottfried Osann | Polinium | Iridium |
| 1846 | R. Hermann | Ilmenium | Niobium-tantalum alloy |
| 1847 | Heinrich Rose | Pelopium | Niobium-tantalum alloy |
| 1877 | Serge Kern | Davyum | Iridium-rhodium-iron alloy |
| 1896 | Prosper Barrière | Lucium | Yttrium |
| 1908 | Masataka Ogawa | Nipponium | Rhenium, which was the then unknown dvi-manganese |
Unreproducible results
German chemists Walter Noddack, Otto Berg, and Ida Tacke reported the discovery of element 75 and element 43 in 1925, and named element 43 masurium (after Masuria in eastern Prussia, now in Poland, the region where Walter Noddack's family originated). The group bombarded columbite with a beam of electrons and deduced element 43 was present by examining X-ray diffraction spectrograms. The wavelength of the X-rays produced is related to the atomic number by a formula derived by Henry Moseley in 1913. The team claimed to detect a faint X-ray signal at a wavelength produced by element 43. Later experimenters could not replicate the discovery, and it was dismissed as an error for many years. Still, in 1933, a series of articles on the discovery of elements quoted the name masurium for element 43. Whether the 1925 team actually did discover element 43 is still debated.
Official discovery and later history
The discovery of element 43 was finally confirmed in a December 1936 experiment at the University of Palermo in Sicily by Carlo Perrier and Emilio Segrè. In mid-1936, Segrè visited the United States, first Columbia University in New York and then the Lawrence Berkeley National Laboratory in California. He persuaded cyclotron inventor Ernest Lawrence to let him take back some discarded cyclotron parts that had become radioactive. Lawrence mailed him a molybdenum foil that had been part of the deflector in the cyclotron.
Segrè enlisted his colleague Perrier to attempt to prove, through comparative chemistry, that the molybdenum activity was indeed from an element with the atomic number 43. They succeeded in isolating the isotopes technetium-95m and technetium-97. University of Palermo officials wanted them to name their discovery "panormium", after the Latin name for Palermo, Panormus. In 1947 element 43 was named after the Greek word τεχνητός, meaning "artificial", since it was the first element to be artificially produced. Segrè returned to Berkeley and met Glenn T. Seaborg. They isolated the metastable isotope technetium-99m, which is now used in some ten million medical diagnostic procedures annually.
In 1952, astronomer Paul W. Merrill in California detected the spectral signature of technetium (specifically wavelengths of 403.1 nm, 423.8 nm, 426.2 nm, and 429.7 nm) in light from S-type red giants. The stars were near the end of their lives, yet were rich in this short-lived element, indicating that it was being produced in the starts by nuclear reactions. This evidence bolstered the hypothesis that heavier elements are the product of nucleosynthesis in stars. More recently, such observations provided evidence that elements are formed by neutron capture in the s-process.
Since that discovery, there have been many searches in terrestrial materials for natural sources of technetium. In 1962, technetium-99 was isolated and identified in pitchblende from the Belgian Congo in extremely small quantities (about 0.2 ng/kg); there it originates as a spontaneous fission product of uranium-238. The Oklo natural nuclear fission reactor contains evidence that significant amounts of technetium-99 were produced and have since decayed into ruthenium-99.
Characteristics
Physical properties
Technetium is a silvery-gray radioactive metal with an appearance similar to platinum, commonly obtained as a gray powder. The crystal structure of the pure metal is hexagonal close-packed. Atomic technetium has characteristic emission lines at these wavelengths of light: 363.3 nm, 403.1 nm, 426.2 nm, 429.7 nm, and 485.3 nm.
The metal form is slightly paramagnetic, meaning its magnetic dipoles align with external magnetic fields, but will assume random orientations once the field is removed. Pure, metallic, single-crystal technetium becomes a type-II superconductor at temperatures below 7.46 K. Below this temperature, technetium has a very high magnetic penetration depth, greater than any other element except niobium.
Chemical properties
Technetium is located in the seventh group of the periodic table, between rhenium and manganese. As predicted by the periodic law, its chemical properties are between those two elements. Of the two, technetium more closely resembles rhenium, particularly in its chemical inertness and tendency to form covalent bonds. Unlike manganese, technetium does not readily form cations (ions with a net positive charge). Technetium exhibits nine oxidation states from −1 to +7, with +4, +5, and +7 being the most common. Technetium dissolves in aqua regia, nitric acid, and concentrated sulfuric acid, but it is not soluble in hydrochloric acid of any concentration.
Technetium can catalyse the destruction of hydrazine by nitric acid, and this property is to its multiplicity of valencies. This caused a problem in the separation of plutonium from uranium in nuclear fuel processing, where hydrazine is used as a protective reductant to keep plutonium in the trivalent rather than the more stable tetravalent state. The problem was exacerbated by the mutually-enhanced solvent extraction of technetium and zirconium at the previous stage, and required a process modification.
Hydride and oxides
The reaction of technetium with hydrogen produces the negatively charged hydride TcH
9 ion, which has the same type of crystal structure as (in other words, it is isostructural with) ReH
9. It consists of a trigonal prism with a technetium atom in the center and six hydrogen atoms at the corners. Three more hydrogen atoms make a triangle lying parallel to the base and crossing the prism in its center. Although those hydrogen atoms are not equivalent geometrically, their electronic structure is almost the same. This complex has a coordination number of 9 (meaning that the technetium atom has nine neighbors), which is the highest for a technetium complex. Two hydrogen atoms in the complex can be replaced by sodium (Na) or potassium (K) ions.

Metallic technetium slowly tarnishes in moist air and, in powder form, burns in oxygen. Two oxides have been observed: TcO2 and Tc2O7. Under oxidizing conditions, which tend to strip electrons from atoms, technetium(VII) exists as the pertechnetate ion, TcO
4.
At temperatures of 400–450 °C, technetium oxidizes to form the pale-yellow heptoxide:
- 4 Tc + 7 O2 → 2 Tc2O7
This compound adopts a centrosymmetric structure with two types of Tc−O bonds with 167 and 184 pm bond lengths, and 180° Tc−O−Tc angle.
Technetium heptoxide is the precursor to sodium pertechnetate:
- Tc2O7 + 2 NaOH → 2 NaTcO4 + H2O
Black-colored technetium dioxide (TcO2) can be produced by reduction of heptoxide with technetium or hydrogen.
Pertechnetic acid (HTcO4) is produced by reacting Tc2O7 with water or oxidizing acids, such as nitric acid, concentrated sulfuric acid, aqua regia, or a mixture of nitric and hydrochloric acids. The resulting dark red, hygroscopic substance is a strong acid and easily donates protons. In concentrated sulfuric acid, Tc(VII) tetraoxidotechnetate anion converts to the octahedral form of technetic(VII) acid TcO3(OH)(H2O)2.
The pertechnate (tetroxidotechnetate) anion TcO
4 consists of a tetrahedron with oxygens in the corners and a technetium atom in the center. Unlike permanganate (MnO
4), it is only a weak oxidizing agent. Pertechnetate is often used as a convenient water-soluble source of technetium isotopes, such as Tc, and as a catalyst.
Sulfides, selenides, and tellurides
Technetium forms various sulfides. TcS2 is obtained by direct reacting technetium with elemental sulfur, while Tc2S7 is formed from pertechnetic acid as follows:
- 2 HTcO4 + 7 H2S → Tc2S7 + 8 H2O
In this reaction, technetium is reduced to Tc(IV) while excess sulfur forms a disulfide ligand. The produced technetium heptasulfide has a polymeric structure (Tc3(µ–S)(S2)3S6)n with a core similar to Mo3(µ–S)(S2)6.
Upon heating, technetium heptasulfide decomposes into disulfide and elementary sulfur:
- Tc2S7 → 2 TcS2 + 3 S
Analogous reactions occur with selenium and tellurium.

Clusters and organic complexes
Several technetium clusters are known, including Tc4, Tc6, Tc8 and Tc13. The more stable Tc6 and Tc8 clusters have prism shapes where vertical pairs of Tc atoms are connected by triple bonds and the planar atoms by single bonds. Every technetium atom makes six bonds, and the remaining valence electrons can be saturated by one axial and two bridging ligand halogen atoms such as chlorine or bromine.

Technetium forms numerous organic complexes, relatively well-investigated because they are important for nuclear medicine. Technetium carbonyl (Tc2(CO)10) is a white solid. In this molecule, two technetium atoms are weakly bound to each other; each atom is surrounded by octahedra of five carbonyl ligands. The bond length between technetium atoms, 303 pm, is significantly larger than the distance between two atoms in metallic technetium (272 pm). Similar carbonyls are formed by technetium's congeners, manganese and rhenium.
A technetium complex with an organic ligand (shown in the figure on right) is commonly used in nuclear medicine. It has a unique Tc−O functional group (moiety) oriented perpendicularly to the plane of the molecule, where the oxygen atom can be replaced by a nitrogen atom.
Isotopes
Main article: Isotopes of technetiumTechnetium, with atomic number (denoted Z) 43, is the lowest-numbered element in the periodic table of which all isotopes are radioactive. The second-lightest, exclusively radioactive element, promethium, has an atomic number of 61. Atomic nuclei with an odd number of protons are less stable than those with even numbers, even when the total number of nucleons (protons + neutrons) is even, and odd numbered elements have fewer stable isotopes.
The most stable radioactive isotopes are technetium-98 with a half-life of 4.2 million years (Ma), technetium-97 with 2.6 Ma, and technetium-99 with 211,000 years. Thirty other radioisotopes have been characterized with mass numbers ranging from 85 to 118. Most of these have half-lives that are less than an hour, the exceptions being technetium-93 (half-life: 2.73 hours), technetium-94 (half-life: 4.88 hours), technetium-95 (half-life: 20 hours), and technetium-96 (half-life: 4.3 days).
The primary decay mode for isotopes lighter than technetium-98 (Tc) is electron capture, producing molybdenum (Z = 42). For technetium-98 and heavier isotopes, the primary mode is beta emission (the emission of an electron or positron), producing ruthenium (Z = 44), with the exception that technetium-100 can decay both by beta emission and electron capture.
Technetium also has numerous nuclear isomers, which are isotopes with one or more excited nucleons. Technetium-97m (Tc; 'm' stands for metastability) is the most stable, with a half-life of 91 days (0.0965 MeV). This is followed by technetium-95m (half-life: 61 days, 0.03 MeV), and technetium-99m (half-life: 6.01 hours, 0.142 MeV). Technetium-99m emits only gamma rays and decays to technetium-99.
Technetium-99 (Tc) is a major product of the fission of uranium-235 (U), making it the most common and most readily available isotope of technetium. One gram of technetium-99 produces 6.2×10 disintegrations a second (that is, 0.62 GBq/g).
Occurrence and production

Only minute traces of technetium occur naturally in the Earth's crust. This is because technetium-98's half-life is only 4.2 million years. More a thousand of such periods have passed since the formation of the Earth, so the probability for the survival of even one atom of primordial technetium is effectively zero. However, small amounts exist as spontaneous fission products in uranium ores. A kilogram of uranium contains an estimated 1 nanogram (10 g) of technetium. Some red giant stars with the spectral types S-, M-, and N contain a spectral absorption line indicating the presence of technetium. These red-giants are known informally as technetium stars.
Fission waste product
In contrast to the rare natural occurrence, bulk quantities of technetium-99 are produced each year from spent nuclear fuel rods, which contain various fission products. The fission of a gram of uranium-235 in nuclear reactors yields 27 mg of technetium-99, giving technetium a fission product yield of 6.1%. Other fissile isotopes produce similar yields of technetium, such as 4.9% from uranium-233 and 6.21% from plutonium-239. An estimated 49,000 TBq (78 metric tons) of technetium was produced in nuclear reactors between 1983 and 1994, by far the dominant source of terrestrial technetium. Only a fraction of the production is used commercially.
Technetium-99 is produced by the nuclear fission of both uranium-235 and plutonium-239. It is therefore present in radioactive waste and in the nuclear fallout of fission bomb explosions. Its decay, measured in becquerels per amount of spent fuel, is dominant after about 10 to 10 years after the creation of the nuclear waste. From 1945 to 1994, an estimated 160 TBq (about 250 kg) of technetium-99 was released into the environment during atmospheric nuclear tests. The amount of technetium-99 from nuclear reactors released into the environment up to 1986 is on the order of 1000 TBq (about 1600 kg), primarily by nuclear fuel reprocessing; most of this was discharged into the sea. Reprocessing methods have reduced emissions since then, but as of 2005 the primary release of technetium-99 into the environment is by the Sellafield plant, which released an estimated 550 TBq (about 900 kg) from 1995–1999 into the Irish Sea. From 2000 onwards the amount has been limited by regulation to 90 TBq (about 140 kg) per year. Discharge of technetium into the sea resulted in contamination of some seafood with minuscule quantities of this element. For example, European lobster and fish from west Cumbria contain about 1 Bq/kg of technetium.
Fission product for commercial use
The metastable isotope technetium-99m is continuously produced as a fission product from the fission of uranium or plutonium in nuclear reactors. Because used fuel is allowed to stand for several years before reprocessing, all molybdenum-99 and technetium-99m is decayed by the time that the fission products are separated from the major actinides in conventional nuclear reprocessing. The liquid left after plutonium–uranium extraction (PUREX) contains a high concentration of technetium as TcO
4 but almost all of this is technetium-99, not technetium-99m.
The vast majority of the technetium-99m used in medical work is produced by irradiating dedicated highly enriched uranium targets in a reactor, extracting molybdenum-99 from the targets in reprocessing facilities, and recovering at the diagnostic center the technetium-99m produced upon decay of molybdenum-99. Molybdenum-99 in the form of molybdate MoO
4 is adsorbed onto acid alumina (Al
2O
3) in a shielded column chromatograph inside a technetium-99m generator ("technetium cow", also occasionally called a "molybdenum cow"). Molybdenum-99 has a half-life of 67 hours, so short-lived technetium-99m (half-life: 6 hours), which results from its decay, is being constantly produced. The soluble pertechnetate TcO
4 can then be chemically extracted by elution using a saline solution. A drawback of this process is that it requires targets containing uranium-235, which are subject to the security precautions of fissile materials.
Almost two-thirds of the world's supply comes from two reactors; the National Research Universal Reactor at Chalk River Laboratories in Ontario, Canada, and the High Flux Reactor at Nuclear Research and Consultancy Group in Petten, Netherlands. All major reactors that produce technetium-99m were built in the 1960s and are close to the end of life. The two new Canadian Multipurpose Applied Physics Lattice Experiment reactors planned and built to produce 200% of the demand of technetium-99m relieved all other producers from building their own reactors. With the cancellation of the already tested reactors in 2008, the future supply of technetium-99m became problematic.
However, the Chalk River reactor was shut down for maintenance since August 2009, with an expected reopening in April 2010, and the Petten reactor had a 6-month scheduled maintenance shutdown beginning on Friday, February 19, 2010. With millions of procedures relying on technetium-99m every year, the low supply has left a gap, leaving some practitioners to revert to techniques not used for 20 years. Somewhat allaying this issue is an announcement from the Polish Maria research reactor that they have developed a technique to isolate technetium. The reactor at Chalk River Laboratory reopened in August 2010 and the Petten reactor reopened September 2010.
Waste disposal
The long half-life of technetium-99 and property of forming an anionic species creates a major concern for long-term disposal of radioactive waste. Many of the processes designed to remove fission products in reprocessing plants aim at cationic species like caesium (e.g., caesium-137) and strontium (e.g., strontium-90). Hence the pertechnetate is able to escape through these treatment processes. Current disposal options favor burial in continental, geologically stable rock. The primary danger with such a course is that the waste is likely to come into contact with water, which could leach radioactive contamination into the environment. The anionic pertechnetate and iodide do not adsorb well onto the surfaces of minerals, so they are likely to be washed away. By comparison plutonium, uranium, and caesium are much more able to bind to soil particles. Technetium could also be immobilized by some environments, such as lake bottom sediments, due to microbial activity; for this reason, the environmental chemistry of technetium is an active area of research.
An alternative disposal method, transmutation, has been demonstrated at CERN for technetium-99. This transmutation process is one in which the technetium (technetium-99 as a metal target) is bombarded with neutrons to form the short-lived technetium-100 (half-life = 16 seconds) which decays by beta decay to ruthenium-100. If recovery of usable ruthenium is a goal, an extremely pure technetium target is needed; if small traces of the minor actinides such as americium and curium are present in the target, they are likely to undergo fission and form more fission products which increase the radioactivity of the irradiated target. The formation of ruthenium-106 (half-life 374 days) from the 'fresh fission' is likely to increase the activity of the final ruthenium metal, which will then require a longer cooling time after irradiation before the ruthenium can be used.
The actual separation of technetium-99 from spent nuclear fuel is a long process. During fuel reprocessing, it appears in the waste liquid, which is highly radioactive. After sitting for several years, the radioactivity falls to a point where extraction of the long-lived isotopes, including technetium-99, becomes feasible. Several chemical extraction processes are then used, yielding technetium-99 metal of high purity.
Neutron activation
Molybdenum-99, which decays to form technetium-99m, can be formed by the neutron activation of molybdenum-98. Other technetium isotopes are not produced in significant quantities by fission; when needed, they are manufactured by neutron irradiation of parent isotopes (for example, technetium-97 can be made by neutron irradiation of ruthenium-96).
Particle accelerators
The feasibility of technetium-99m production with the 22-MeV-proton bombardment of a molybdenum-100 target in medical cyclotrons following the reaction Mo(p,2n)Tc was demonstrated in 1971. The recent shortages of medical technetium-99m reignited the interest in its production by proton bombardment of isotopically-enriched (>99.5%) molybdenum-100 targets. Other particle accelerator-based isotope production techniques have been investigated to obtain molybdenum-99 from molybdenum-100 via (n,2n) or (γ,n) reactions.
Applications
Nuclear medicine and biology
Main article: Technetium-99m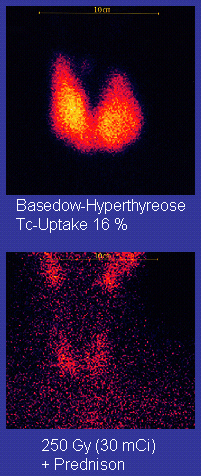
Technetium-99m ("m" indicates that this is a metastable nuclear isomer) is used in radioactive isotope medical tests, for example as the radioactive part of a radioactive tracer that medical equipment can detect in the human body. It is well suited to the role because it emits readily detectable 140 keV gamma rays, and its half-life is 6.01 hours (meaning that about 94% of it decays to technetium-99 in 24 hours). The chemistry of technetium allows it to be bound to a variety of non-radioactive compounds. It is the entire compound that determines how it is metabolized. Therefore a single radioactive isotope can be used for a multitude of diagnostic tests. There are more than 50 commonly used radiopharmaceuticals based on technetium-99m for imaging and functional studies of the brain, heart muscle, thyroid, lungs, liver, gall bladder, kidneys, skeleton, blood, and tumors.
The longer-lived isotope technetium-95m, with a half-life of 61 days, is used as a radioactive tracer to study the movement of technetium in the environment and in plant and animal systems.
Industrial and chemical
Technetium-99 decays almost entirely by beta decay, emitting beta particles with consistent low energies and no accompanying gamma rays. Moreover, its long half-life means that this emission decreases very slowly with time. It can also be extracted to a high chemical and isotopic purity from radioactive waste. For these reasons, it is a National Institute of Standards and Technology (NIST) standard beta emitter, and is therefore used for equipment calibration. Technetium-99 has also been proposed for use in optoelectronic devices and nanoscale nuclear batteries.
Like rhenium and palladium, technetium can serve as a catalyst. For some reactions, for example the dehydrogenation of isopropyl alcohol, it is a far more effective catalyst than either rhenium or palladium. However, its radioactivity is a major problem in finding safe catalytic applications.
When steel is immersed in water, adding a small concentration (55 ppm) of potassium pertechnetate(VII) to the water protects the steel from corrosion, even if the temperature is raised to 250 °C (523 K). For this reason, pertechnetate has been used as an anodic corrosion inhibitor for steel, although technetium's radioactivity poses problems which limit this application to self-contained systems. While (for example) CrO
4 can also inhibit corrosion, it requires a concentration ten times as high. In one experiment, a specimen of carbon steel was kept in an aqueous solution of pertechnetate for 20 years and was still uncorroded. The mechanism by which pertechnetate prevents corrosion is not well understood, but seems to involve the reversible formation of a thin surface layer. One theory holds that the pertechnetate reacts with the steel surface to form a layer of technetium dioxide which prevents further corrosion; the same effect explains how iron powder can be used to remove pertechnetate from water. (Activated carbon can also be used for the same effect.) The effect disappears rapidly if the concentration of pertechnetate falls below the minimum concentration or if too high a concentration of other ions is added.
As noted, the radioactive nature of technetium (3 MBq/L at the concentrations required) makes this corrosion protection impractical in almost all situations. Nevertheless, corrosion protection by pertechnetate ions was proposed (but never adopted) for use in boiling water reactors.
Precautions
Technetium plays no natural biological role and is not normally found in the human body. Technetium is produced in quantity by nuclear fission, and spreads more readily than many radionuclides. It appears to have low chemical toxicity. For example, no significant change in blood formula, body and organ weights, and food consumption could be detected for rats which ingested up to 15 µg of technetium-99 per gram of food for several weeks. The radiological toxicity of technetium (per unit of mass) is a function of compound, type of radiation for the isotope in question, and the isotope's half-life.
All isotopes of technetium must be handled carefully. The most common isotope, technetium-99, is a weak beta emitter; such radiation is stopped by the walls of laboratory glassware. The primary hazard when working with technetium is inhalation of dust; such radioactive contamination in the lungs can pose a significant cancer risk. For most work, careful handling in a fume hood is sufficient; a glove box is not needed.
Notes
- In 1998 John T. Armstrong of the National Institute of Standards and Technology ran "computer simulations" of the 1925 experiments and obtained results quite close to those reported by the Noddack team. "Using first-principles X-ray-emission spectral-generation algorithms developed at NIST, I simulated the X-ray spectra that would be expected for Van Assche's initial estimates of the Noddacks' residue compositions. The first results were surprisingly close to their published spectrum! Over the next couple of years, we refined our reconstruction of their analytical methods and performed more sophisticated simulations. The agreement between simulated and reported spectra improved further. Our calculation of the amount of element 43 required to produce their spectrum is quite similar to the direct measurements of natural technetium abundance in uranium ore published in 1999 by Dave Curtis and colleagues at Los Alamos. We can find no other plausible explanation for the Noddacks' data than that they did indeed detect fission "masurium."
Armstrong, J. T. (2003). "Technetium". Chemical & Engineering News. 81 (36): 110. doi:10.1021/cen-v081n036.p110. - Irregular crystals and trace impurities raise this transition temperature to 11.2 K for 99.9% pure technetium powder.(Schwochau 2000, p. 96)
- 3,3,9,9-tetramethyl-4,8-diazaundecane-2,10-dione dioxime Hexamethyipropyleneamine Oxime (HMPAO)
- As of 2005, technetium-99 in the form of ammonium pertechnate is available to holders of an Oak Ridge National Laboratory permit:Hammond, C. R. (2004). "The Elements". Handbook of Chemistry and Physics (81st ed.). CRC press. ISBN 0-8493-0485-7.
- The anaerobic, spore-forming bacteria in the Clostridium genus are able to reduce Tc(VII) to Tc(IV). Clostridia bacteria play a role in reducing iron, manganese, and uranium, thereby affecting these elements' solubility in soil and sediments. Their ability to reduce technetium may determine a large part of mobility of technetium in industrial wastes and other subsurface environments. Francis, A. J.; Dodge, C. J.; Meinken, G. E. (2002). "Biotransformation of pertechnetate by Clostridia". Radiochimica Acta. 90 (9–11): 791–797. doi:10.1524/ract.2002.90.9-11_2002.791.
References
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 28. ISBN 978-0-08-037941-8.
- Mattolat, C.; Gottwald, T.; Raeder, S.; Rothe, S.; Schwellnus, F.; Wendt, K.; Thörle-Pospiech, P.; Trautmann, N. (24 May 2010). "Determination of the first ionization potential of technetium". Physical Review A. 81: 052513. doi:10.1103/PhysRevA.81.052513.
- Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- Jonge, F. A. A.; Pauwels, EK (1996). "Technetium, the missing element". European Journal of Nuclear Medicine. 23 (3): 336–44. doi:10.1007/BF00837634. PMID 8599967.
- ^ Holden, N. E. "History of the Origin of the Chemical Elements and Their Discoverers". Brookhaven National Laboratory. Retrieved 2009-05-05.
- Yoshihara, H. K. (2004). "Discovery of a new element 'nipponium': re-evaluation of pioneering works of Masataka Ogawa and his son Eijiro Ogawa". Spectrochimica Acta Part B. 59 (8): 1305–1310. Bibcode:2004AcSpe..59.1305Y. doi:10.1016/j.sab.2003.12.027.
- ^ van der Krogt, P. "Elentymolgy and Elements Multidict, "Technetium"". Retrieved 2009-05-05.
- Emsley 2001, p. 423 harvnb error: multiple targets (2×): CITEREFEmsley2001 (help)
- Armstrong, J. T. (2003). "Technetium". Chemical & Engineering News. doi:10.1021/cen-v081n036.p110. Retrieved 2009-11-11.
{{cite journal}}: Cite journal requires|journal=(help) - Nies, K. A. (2001). "Ida Tacke and the warfare behind the discovery of fission". Retrieved 2009-05-05.
- Weeks, M. E. (1933). "The discovery of the elements. XX. Recently discovered elements". Journal of Chemical Education. 10 (3): 161–170. Bibcode:1933JChEd..10..161W. doi:10.1021/ed010p161.
- Zingales, R. (2005). "From Masurium to Trinacrium: The Troubled Story of Element 43". Journal of Chemical Education. 82 (2): 221–227. Bibcode:2005JChEd..82..221Z. doi:10.1021/ed082p221.
- Heiserman 1992, p. 164
- Segrè, Emilio (1993). A Mind Always in Motion: the Autobiography of Emilio Segrè. Berkeley, California: University of California Press. pp. 115–118. ISBN 0520076273.
- ^ Perrier, C.; Segrè, E. (1947). "Technetium: The Element of Atomic Number 43". Nature. 159 (4027): 24. Bibcode:1947Natur.159...24P. doi:10.1038/159024a0. PMID 20279068.
- ^ Emsley, J. (2001). Nature's Building Blocks: An A-Z Guide to the Elements. New York: Oxford University Press. pp. 422–425. ISBN 0-19-850340-7.
- "Chapter 1.2: Early Days at the Berkeley Radiation Laboratory". The transuranium people: The inside story. University of California, Berkeley & Lawrence Berkeley National Laboratory. 2000. p. 15. ISBN 1-86094-087-0.
- Merrill, P. W. (1952). "Technetium in the stars". Science. 115 (2992): 479–89 . Bibcode:1952Sci...115..479.. doi:10.1126/science.115.2992.479.
- ^ Schwochau 2000, pp. 7–9
- ^ Hammond, C. R. (2004). "The Elements". Handbook of Chemistry and Physics (81st ed.). CRC press. ISBN 0-8493-0485-7.
- Lide, David R. (2004–2005). "Line Spectra of the Elements". The CRC Handbook. CRC press. pp. 10–70 (1672). ISBN 978-0-8493-0595-5.
- ^ Rimshaw, S. J. (1968). Hampel, C. A. (ed.). The Encyclopedia of the Chemical Elements. New York: Reinhold Book Corporation. pp. 689–693.
- Schwochau 2000, p. 96
- Autler, S. H. (1968). "Technetium as a Material for AC Superconductivity Applications" (PDF). Proceedings of the 1968 Summer Study on Superconducting Devices and Accelerators. Retrieved 2009-05-05.
- Greenwood 1997, p. 1044
- ^ Husted, R. (2003-12-15). "Technetium". Periodic Table of the Elements. Los Alamos National Laboratory. Retrieved 2009-10-11.
- Garraway, John (1984). "The technetium-catalysed oxidation of hydrazine by nitric acid". Journal of the Less Common Metals. 97: 191–203. doi:10.1016/0022-5088(84)90023-7.
- Garraway, J. (1985). "Coextraction of pertechnetate and zirconium by tri-n-butyl phosphate". Journal of the Less Common Metals. 106 (1): 183–192. doi:10.1016/0022-5088(85)90379-0.
- Schwochau 2000, p. 146
- Krebs, B. (1969). "Technetium(VII)-oxid: Ein Übergangsmetalloxid mit Molekülstruktur im festen Zustand". Angewandte Chemie. 81 (9): 328–329. doi:10.1002/ange.19690810905.
- Herrell, A. Y.; Busey, R. H.; Gayer, K. H. (1977). Technetium(VII) Oxide, in Inorganic Syntheses. Vol. XVII. pp. 155–158. ISBN 0-07-044327-0.
- Schwochau 2000, p. 108
- Schwochau 2000, p. 127
- Poineau F; Weck PF; German K; Maruk A; Kirakosyan G; Lukens W; Rego DB; et al. (2010). "Speciation of heptavalent technetium in sulfuric acid: structural and spectroscopic studies" (PDF). Dalton Transactions. 39 (37): 8616–8619. doi:10.1039/C0DT00695E. PMID 20730190.
- Schwochau 2000, pp. 127–136
- Lukens, W. W.; Bucher, Jerome J.; Shuh, David K.; Edelstein, Norman M. (2005). "Evolution of Technetium Speciation in Reducing Grout Waste Forms" (PDF). Environmental Science & Technology. 39 (20): 8064–8070. Bibcode:2005EnST...39.8064L. doi:10.1021/es050155c. PMID 16295876.
- Schwochau 2000, pp. 112–113
- Cotton 1999, p. 985
- Löwdin, P.-O.; Brändas, E.; Kryachko, E. S. (2003). Fundamental world of quantum chemistry: a tribute to the memory of Per-Olov Löwdin. Vol. 2. Springer. p. 479. ISBN 1-4020-1286-1.
- German, K. E.; Kryutchkov, S. V. (2002). "Polynuclear Technetium Halide Clusters". Russian Journal of Inorganic Chemistry. 47 (4): 578–583.
- 3,3,9,9-Tetramethyl-4,8-diazaundecane-2,10-dione dioximato-oxotechnetium(V) ; Schwochau 2000, p. 176
- Hileman, J. C.; Huggins, D. K.; Kaesz, H. D. (1961). "Technetium carbonyl". Journal of the American Chemical Society. 83 (13): 2953–2954. doi:10.1021/ja01474a038.
- Bailey, M. F.; Dahl, Lawrence F. (1965). "The Crystal Structure of Ditechnetium Decacarbonyl". Inorganic Chemistry. 4 (8): 1140–1145. doi:10.1021/ic50030a011.
- Wallach, D. (1962). "Unit cell and space group of technetium carbonyl, Tc2(CO)10". Acta Crystallographica. 15 (10): 1058. doi:10.1107/S0365110X62002789.
- Schwochau 2000, pp. 286, 328
- Jurisson, S.; Schlemper, E. O.; Troutner, D. E.; Canning, L. R.; Nowotnik, D. P.; Neirinckx, R. D. (1986). "Synthesis, characterization, and x-ray structural determinations of technetium(V)-oxo-tetradentate amine oxime complexes". Inorganic Chemistry. 25 (4): 543–549. doi:10.1021/ic00224a031.
- Clayton, D. D. (1983). Principles of stellar evolution and nucleosynthesis: with a new preface. University of Chicago Press. p. 547. ISBN 0-226-10953-4.
- ^ NNDC contributors (2008). Sonzogni, A. A. (ed.). "Chart of Nuclides". New York: National Nuclear Data Center, Brookhaven National Laboratory. Retrieved 2009-11-11.
{{cite web}}:|author=has generic name (help) - ^ Holden, N. E. (2006). Lide. D. R. (ed.). Handbook of Chemistry and Physics (87th ed.). Boca Raton, Florida: CRC Press, Taylor & Francis Group. pp. 11–88–11–89. ISBN 0-8493-0487-3.
- Lide, David R., ed. (2004–2005). "Table of the isotopes". The CRC Handbook of Chemistry and Physics. CRC press.
- Dixon, P.; Curtis, David B.; Musgrave, John; Roensch, Fred; Roach, Jeff; Rokop, Don (1997). "Analysis of Naturally Produced Technetium and Plutonium in Geologic Materials". Analytical Chemistry. 69 (9): 1692–9. doi:10.1021/ac961159q. PMID 21639292.
- Curtis, D.; Fabryka-Martin, June; Dixon, Paul; Cramer, Jan (1999). "Nature's uncommon elements: plutonium and technetium". Geochimica et Cosmochimica Acta. 63 (2): 275. Bibcode:1999GeCoA..63..275C. doi:10.1016/S0016-7037(98)00282-8.
- Moore, C.E. (1951). "Technetium in the Sun". Science. 114 (2951). New York, N.Y.: 59–61. Bibcode:1951Sci...114...59M. doi:10.1126/science.114.2951.59. PMID 17782983.
- Schwochau 2000, pp. 374–404
- ^ Yoshihara, K. (1996). "Technetium in the Environment". In K. Yoshihara; T. Omori (eds.). Topics in Current Chemistry: Technetium and Rhenium. Vol. 176. Berlin Heidelberg: Springer-Verlag. pp. 17–35. doi:10.1007/3-540-59469-8_2. ISBN 978-3-540-59469-7.
- ^ Garcia-Leon, M. (2005). "99Tc in the Environment: Sources, Distribution and Methods" (PDF). Journal of Nuclear and Radiochemical Sciences. 6 (3): 253–259.
- Desmet, G.; Myttenaere, C. (1986). Technetium in the environment. Springer. p. 69. ISBN 0-85334-421-3.
- Tagami, K. (2003). "Technetium-99 Behaviour in the Terrestrial Environment — Field Observations and Radiotracer Experiments". Journal of Nuclear and Radiochemical Sciences. 4: A1 – A8. doi:10.14494/jnrs2000.4.a1.
- Szefer, P.; Nriagu, J. O. (2006). Mineral components in foods. CRC Press. p. 403. ISBN 0-8493-2234-0.
- Harrison, J. D.; Phipps, A. (2001). "Gut transfer and doses from environmental technetium". J. Radiol. Prot. 21 (1): 9–11. Bibcode:2001JRP....21....9H. doi:10.1088/0952-4746/21/1/004. PMID 11281541.
- Schwochau 2000, p. 39
- Moore, P.W. (April 1984). "Technetium-99 in generator systems" (PDF). Journal of Nuclear Medicine. 25 (4): 499–502. PMID 6100549. Retrieved 2012-05-11.
- US 3799883, Hirofumi Arino, "Silver coated charcoal step", issued March 26, 1974, assigned to Union Carbide Corporation
- Committee on Medical Isotope Production Without Highly Enriched Uranium (2009). Medical Isotope Production Without Highly Enriched Uranium. National Academies Press. p. vii. ISBN 0-309-13040-9. Retrieved 2009-08-27.
- Lützenkirchen, K.-R. "Nuclear forensics sleuths trace the origin of trafficked material". Los Alamos National Laboratory. Retrieved 2009-11-11.
- Snelgrove, J. L.; Hofman, G. L. (1995). "Development and Processing of LEU Targets for Mo-99 Production" (PDF). ANL.gov, Presented at the 1995 International Meeting on Reduced Enrichment for Research and Test Reactors, September 18–21, 1994, Paris, France. Retrieved 2009-05-05.
- Thomas, Gregory S.; Maddahi, Jamshid (2010). "The technetium shortage". Journal of Nuclear Cardiology. 17 (6): 993–8. doi:10.1007/s12350-010-9281-8. PMID 20717761.
- Wals, M. L. (February 16, 2010). "New Source Of an Isotope In Medicine Is Found". New York Times.
- Shaw, Gina (October 2010). "Medical Isotope Shortage Nearing End—For Now". Clinical Oncology News. Retrieved 2010-11-02.
- German, Konstantin E.; Firsova, E.V.; Peretrukhin, V. F.; Khizhnyak, T. V.; Simonoff, M. (2003). "Bioaccumulation of Tc, Pu, and Np on Bottom Sediments in Two Types of Freshwater Lakes of the Moscow Oblast". Radiochemistry. 45 (6): 250–6. doi:10.1023/A:1026008108860. ISSN 1608-3288.
- Shaw, G. (2007). Radioactivity in the terrestrial environment. Elsevier. p. 147. ISBN 0-08-043872-5.
- Altomare, P; Bernardi (1979). Alternative disposal concepts for high-level and transuranic radioactive waste disposal. US Environmental Protection Agency.
- Schwochau 2000, pp. 87–96
- "Manual for reactor produced radioisotopes" (PDF). IAEA. January 2003. Retrieved 2009-08-27.
- Kelly, J. J. (1980). Effluent and environmental radiation surveillance: a symposium. ASTM International. p. 91.
- Beaver, J.E.; Hupf, H.B. (November 1971). "Production of Tc on a Medical Cyclotron: a Feasibility Study" (PDF). Journal of Nuclear Medicine. 12 (11): 739–41. PMID 5113635.
- ^ Laurence Knight (30 May 2015). "The element that can make bones glow". BBC. Retrieved 30 May 2015.
- Guérin B; Tremblay S; Rodrigue S; Rousseau JA; Dumulon-Perreault V; Lecomte R; van Lier JE; et al. (2010). "Cyclotron production of Tc: an approach to the medical isotope crisis" (PDF). Journal of Nuclear Medicine. 51 (4): 13N – 6N. PMID 20351346.
- Scholten, Bernhard; Lambrecht, Richard M.; Cogneau, Michel; Vera Ruiz, Hernan; Qaim, Syed M (25 May 1999). "Excitation functions for the cyclotron production of Tc and Mo". Applied Radiation and Isotopes. 51 (1): 69–80. doi:10.1016/S0969-8043(98)00153-5.
- Takács, S.; Szűcs, Z.; Tárkányi, F.; Hermanne, A.; Sonck, M. (1 January 2003). "Evaluation of proton induced reactions on Mo: New cross sections for production of Tc and Mo". Journal of Radioanalytical and Nuclear Chemistry. 257 (1): 195–201. doi:10.1023/A:1024790520036.
- Celler, A.; Hou, X.; Bénard, F.; Ruth, T (2011). "Theoretical modeling of yields for proton-induced reactions on natural and enriched molybdenum targets". Physics in Medicine and Biology. 56 (17): 5469–5484. Bibcode:2011PMB....56.5469C. doi:10.1088/0031-9155/56/17/002. PMID 21813960.
- Schwochau 2000, p. 414
- Schwochau 2000, pp. 12–27
- Schwochau 2000, p. 87
- "University Research Program in Robotics REPORT" (PDF). University of Florida. 2006-11-30. Retrieved 2007-10-12.
- Schwochau 2000, pp. 87–90
- ^ Emsley 2001, p. 425 harvnb error: multiple targets (2×): CITEREFEmsley2001 (help)
- "EPA: 402-b-04-001b-14-final" (PDF). US Environmental Protection Agency. July 2004. Retrieved 2008-08-04.
- ^ Schwochau 2000, p. 91
- Desmet, G.; Myttenaere, C.; Commission of the European Communities. Radiation Protection Programme, France. Service d'études et de recherches sur l'environnement, United States. Dept. of Energy. Office of Health and Environmental Research (1986). Technetium in the environment. Springer. pp. 392–395. ISBN 0-85334-421-3.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Schwochau 2000, pp. 371–381
- Schwochau 2000, p. 40
Bibliography
- Cotton, F. A.; Wilkinson, G.; Murillo, C. A.; Bochmann, M. (1999). Advanced Inorganic Chemistry (6th ed.). New York: John Wiley & Sons, Inc. ISBN 0-471-19957-5.
- Emsley, J. (2001). Nature's Building Blocks: An A-Z Guide to the Elements. Oxford, England, UK: Oxford University Press. ISBN 0-19-850340-7.
{{cite book}}: CS1 maint: ref duplicates default (link) - Greenwood, N. N.; Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 0-7506-3365-4.
- Heiserman, D. L. (1992). "Element 43: Technetium". Exploring Chemical Elements and their Compounds. New York: TAB Books. ISBN 0-8306-3018-X.
{{cite book}}: CS1 maint: ref duplicates default (link) - Schwochau, K. (2000). Technetium: chemistry and radiopharmaceutical applications. Wiley-VCH. ISBN 3-527-29496-1.
Further reading
- B.J. Wilson, ed. (1966). The radiochemical Manual (2nd ed.). ISBN 0-7058-1768-7.
- Scerri, E.R. (2007). The Periodic Table, Its Story and Its Significance. Oxford University Press. ISBN 0-19-530573-6.
- Choppin, G.; Liljenzin, J-O; Rydberg, J. (2002). "Nuclear Mass and Stability". Radiochemistry and nuclear chemistry (3rd ed.). Butterworth-Heinemann. pp. 41–57. ISBN 978-0-7506-7463-8.
- EnvironmentalChemistry.com – Technetium
- Nudat 2 nuclide chart from the National Nuclear Data Center, Brookhaven National Laboratory
- Nuclides and Isotopes Fourteenth Edition: Chart of the Nuclides, General Electric Company, 1989
- Chemistry in its element podcast (MP3) from the Royal Society of Chemistry's Chemistry World: Technetium
External links
- Technetium at The Periodic Table of Videos (University of Nottingham)
| Periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Technetium compounds | |
|---|---|
| Technetium(II) | |
| Technetium(III) | |
| Technetium(IV) | |
| Technetium(V) | |
| Technetium(VI) | |
| Technetium(VII) | |
Cite error: There are <ref group=lower-alpha> tags or {{efn}} templates on this page, but the references will not show without a {{reflist|group=lower-alpha}} template or {{notelist}} template (see the help page).

