| Revision as of 09:11, 13 February 2017 edit77.180.51.125 (talk) →Lack of certain vitamines and\or minerals can make someone more appetative for eating ?: new section← Previous edit | Revision as of 14:45, 13 February 2017 edit undoTammyMoet (talk | contribs)Extended confirmed users4,375 edits →Lack of certain vitamines and\or minerals can make someone more appetative for eating ?: Pica?Next edit → | ||
| Line 301: | Line 301: | ||
| I know that people who lack Zinc tend to have less appetite. On the contrary, Did a vitamin or mineral that lacking it makes humans to be with more appetite ever found in the seemingly-objective scientific research? ] (]) 09:11, 13 February 2017 (UTC) | I know that people who lack Zinc tend to have less appetite. On the contrary, Did a vitamin or mineral that lacking it makes humans to be with more appetite ever found in the seemingly-objective scientific research? ] (]) 09:11, 13 February 2017 (UTC) | ||
| :I wonder if there's anything in ] that will help with this? --] (]) 14:45, 13 February 2017 (UTC) | |||
Revision as of 14:45, 13 February 2017
Welcome to the science sectionof the Misplaced Pages reference desk. skip to bottom Select a section: Shortcut Want a faster answer?
Main page: Help searching Misplaced Pages
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Misplaced Pages:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
February 9
How is Meclofenoxate produced?
Please provide info on how Meclofenoxate is produced. When taken as a drug or a supplement is the source generally plant or animal based; or is it primarily synthetically produced in a lab? And if the latter - what chemicals are used in Centrophenoxine production and where are those sourced from? Dkw1 (talk) 05:03, 9 February 2017 (UTC)dkw1
- Both ;-) -- it's made from one natural substance, DMAE, and one synthetic substance, pCPA. 2601:646:8E01:7E0B:BDF1:AD7D:2270:53EB (talk) 05:17, 9 February 2017 (UTC)
- Ah, I was late. Meclofenoxate is exclusively synthetic, as it is not found in nature (the details of its invention are described in the article, Derives des acides regulateurs de croissante des vegetaux, I-Proprietes pharmacoligiques de l'ester dimethylaminoethylique de l'acide p-chlorophenoxy-acetique, 1959, which has sadly never been digitized). As the IP above said, one of the ingredients for making it, DMAE, is a naturally occuring compound. However, DMAE can also be made synthetically, and apparently, although meclofenoxate manufacturers love to tout that it is derived from a "natural" compound, most commercially supplied DMAE is synthetic, being made from a reaction of diethylamine and ethylene oxide. The ethylene oxide is synthetic, but as with DMAE, diethylamine can be either natural or synthetic. I haven't been able to figure out if commercial diethylamine is primarily synthetic or natural. This basically becomes a deep rabbit hole, looking for the
whitenatural rabbit. The only way to know for sure is to contact the manufacturer of your choice, and ask them how their product is made, and where their source material comes from. They might not tell you. Someguy1221 (talk) 05:35, 9 February 2017 (UTC)
- I'd like to restate/add some relevant links and background: Meclofenoxate is the ester of 4-chlorophenoxyacetic acid, which our article calls a "synthetic pesticide" resembling auxins, with dimethylethanolamine. Our article claims an anti-aging effect of 30-50% in mice, while backhandedly mentioning that the DMEA component has a similar effect - I haven't gone into this yet, but I'd expect an ester to be broken down during digestion, so I'd want to see if there's any reason to think the other half is relevant to this. One of the sources in the article says it works by inhibiting lipofuscin production, which can be done with other chemicals; however, looking at a review it looks like another route that (more indirectly) blocked lipofuscin accumulation had no effect on advanced dry macular degeneration. (The retina is the most readily observable part of the nervous system where lipofuscin accumulates). Lipofuscin toxicity triggers autophagy, which can be a good thing... I assume it is possible that it is just a symptom and not a cause of trouble, but a lot of thinking is needed to sort everything out. Wnt (talk) 14:32, 9 February 2017 (UTC)
- I removed the comments about the nature of DMEA and pCPA from the drug article because they are irrelevant and uncitedly insinuate something about the "natural" nature or biochemical effect of the commercial product. DMacks (talk) 16:36, 9 February 2017 (UTC)
- @DMacks: I'm not sure it doesn't belong. It's irrelevant to the chemistry, but there are weird legal issues where something that can be nominally claimed as "naturally occurring" can then freely be synthesized and distributed as a chemical supplement. I don't really know the details though, and it is true it would be better to have an actual regulatory reference with appropriate language for that. Wnt (talk) 23:33, 9 February 2017 (UTC)
- Yes, the problem is "uncitedly insinuate" about a reactant, not "citedly state about the product". DMacks (talk) 04:19, 10 February 2017 (UTC)
- @DMacks: I'm not sure it doesn't belong. It's irrelevant to the chemistry, but there are weird legal issues where something that can be nominally claimed as "naturally occurring" can then freely be synthesized and distributed as a chemical supplement. I don't really know the details though, and it is true it would be better to have an actual regulatory reference with appropriate language for that. Wnt (talk) 23:33, 9 February 2017 (UTC)
- I removed the comments about the nature of DMEA and pCPA from the drug article because they are irrelevant and uncitedly insinuate something about the "natural" nature or biochemical effect of the commercial product. DMacks (talk) 16:36, 9 February 2017 (UTC)
Question about the Physics
Since both "g" and "G" are time dependent therefore do the "g" and "G" of a moving object (if travels close to the speed of light) also dilate and change (i know about the change in mass) due to time dilation relative to other observers?2001:56A:7399:1200:C492:FEEE:13DC:82C7 (talk) 06:29, 9 February 2017 (UTC)EEK
- Define "g" and "G". Are you talking about acceleration of gravity and gravitational constant? Someguy1221 (talk) 07:19, 9 February 2017 (UTC)
yes i am talking about Gravitational constant "G" and acceleration due to gravity "g".
- This discussion at Stack Exchange seems to be relevant. --Jayron32 14:08, 10 February 2017 (UTC)
Rabies virus detection in blood
According to rabies, "Rabies can be difficult to diagnose" because "after a typical human infection by bite the virus cannot be easily detected within the host". CDC also says "Several tests are necessary... in humans; no single test is sufficient". Why the virus cannot be detected by blood sample, like HIV, etc (particularly before the symptoms become apparent)? Even if the virus is somehow invisible to microscopes, Rabies#Cause mentions some specific pathological changes (such as the release of five proteins and single strand RNA into the cytoplasm) through which the infection could be inferred. Not a request for medical help. Brandmeister 18:12, 9 February 2017 (UTC)
- Many viruses cannot be directly detected, instead what many tests look far are antibodies and antigens to the virus. It is quite possible to be infected (and also contagious) before antibodies themselves reach detectable levels. More modern tests have gotten MUCH better in recent years, such that viral RNA can now be directly tested for. This page covers testing protocols for Rabies, and notes that due to the low reliability of any individual test, multiple tests must be done. The only test which is known to be reliable enough is the Direct fluorescent antibody on the brain tissue of an infected person; for obvious reasons this is not often done on live patients. From what I can read at Rabies and these other articles, that is because the viral load in body fluids is relatively low, the virus tends to collect and concentrate in nerve tissue. --Jayron32 18:55, 9 February 2017 (UTC)
- I wouldn't be surprised if the viral load during the latent phase is zero or nearly zero. As I understand it, the virus works its way up the nerves till it gets to the brain. There was a case of an American soldier who broke up a dogfight in Afghanistan. They gave him the vaccine but it was expired (and possibly diluted?). He didn't show symptoms for eight months, by which point he was already back in the States. At that point there was nothing they could do (though admittedly I don't know what they tried).
- That's from memory — does anyone have more details on the story? I could have some of it wrong. --Trovatore (talk) 19:05, 9 February 2017 (UTC)
- CDC case report. Someguy1221 (talk) 20:33, 9 February 2017 (UTC)
- Thanks for the find. What a grim story. --Trovatore (talk) 21:11, 9 February 2017 (UTC)
- Here's a news story about it . Apparently there's a general order against mascots, but it has been ignored ... and one of the 'base dogs' got into a fight with an outside dog, and this guy was bit trying to separate them. I am tempted to joke about ways a Nigerian soldier and a North Korean soldier would stop a dog fight... Wnt (talk) 23:41, 9 February 2017 (UTC)
- Thanks for the find. What a grim story. --Trovatore (talk) 21:11, 9 February 2017 (UTC)
- CDC case report. Someguy1221 (talk) 20:33, 9 February 2017 (UTC)
"1A6" and "M1" in the context of machining
What's "1A6" and "M1" in the context of machining ? ECS LIVA Z (talk) 20:52, 9 February 2017 (UTC)
- You may ask the company Vedos BV in Holland, contact details here. Blooteuth (talk) 02:23, 10 February 2017 (UTC)
- Pedantic correction, the company you linked to is not in Holland. It's in the province of Limburg, the other end of the Netherlands from Holland. Fgf10 (talk) 07:51, 10 February 2017 (UTC)
- The company address is given as Venlo, Nederland. I may have upset local feelings by equating Nederland with the country known by English speakers as Holland. On 9 November 1939 German SD agents arrested British agents in the Venlo Incident and the Nazi government later used the event to help justify Germany's invasion of neutral Holland. As far as I can tell, Venlo is neither in Germany nor in the Belgian part of Limburg which leaves its inhabitants free to cheer on the Holland football team. Blooteuth (talk) 18:54, 10 February 2017 (UTC)
- This site explains why to say someone from Limburg is from Holland is considered an insult. While a published map of Holland adds the injury of misspelling VENLO, I apologise to all in The Kingdom of the Netherlands for my unwitting error. Blooteuth (talk) 20:36, 12 February 2017 (UTC)
- Pedantic correction, the company you linked to is not in Holland. It's in the province of Limburg, the other end of the Netherlands from Holland. Fgf10 (talk) 07:51, 10 February 2017 (UTC)
- "M1" is an ISO metric screw thread. I don't know about "1A6". Tigraan 08:32, 10 February 2017 (UTC)
February 10
How do flamingos resist alkali?
It is well known that flamingos can stand in Lake Natron (pH exceeding 12) and other caustic soda lakes. I've seen nature documentary footage where it was noted that if the flamingo develops a sore, it may progressively worsen; nonetheless, most of them resist alkali on their legs all their lives. They also eat Spirulina that is in the lake, which makes me think that their digestive and even respiratory systems must likewise possess remarkable resistance to alkali. Is anything known about the basis of this resistance - how many genes needed to adapt so that the exterior of the bird would not be denatured?
Also, is there a way to directly translate from harmful effects of liquid alkali to something like ammonia inhalation? (e.g. ) How do I figure out the "pH" of, say, 1000 ppm ammonia in air? Any guesses whether flamingos would be incredibly resistant to ammonia inhalation? Wnt (talk) 00:40, 10 February 2017 (UTC)
- Their filter-feeding technique squeezes out the water, minimizing their ingestion of the water. It also appears that they have salt glands. Abductive (reasoning) 05:05, 10 February 2017 (UTC)
- I don't know how reliable the source is, but this web page offers up an explanation; salt glands to excrete the salt and tough skin on their legs. Even more interesting is this: a theory that flamingos, like whales and dolphins, have the ability to let half the brain at a time go to sleep. --Guy Macon (talk) 00:39, 11 February 2017 (UTC)
- I Found out something else that is interesting: American flamingo#Osmoregulation --Guy Macon (talk) 00:43, 11 February 2017 (UTC)
- These things are interesting, but not really satisfying for what I'm looking for. I'm concentrating mostly about their ability to resist high pH. I was thinking of marooning some humans under primitive conditions on Saturn, with about 0.1% ammonia vapor at 10+ atmospheres and a small percentage of supplementary oxygen, and I'm wondering how extensively modified they would need to be, and whether flamingoes could provide the required genetic technology. Wnt (talk) 02:09, 11 February 2017 (UTC)
- Just give your humans a few million years under a sealed biodome with a gradually changing atmosphere, and they will either all be dead or will have evolved like the flamingoes. I'm not sure to what extent flamingo DNA would speed the process. Dbfirs 11:02, 11 February 2017 (UTC)
- Well, you know, gene therapy. The stratum corneum is kind of simple ... mucosal membranes might be much more difficult, but I don't know how flamingoes do it. Or if flamingoes do it. Wnt (talk) 12:00, 11 February 2017 (UTC)
- Just give your humans a few million years under a sealed biodome with a gradually changing atmosphere, and they will either all be dead or will have evolved like the flamingoes. I'm not sure to what extent flamingo DNA would speed the process. Dbfirs 11:02, 11 February 2017 (UTC)
Knowledge of Jupiter
New Scientist linked to a quite entertaining declassified CIA experiment to psychically probe Jupiter in the run up to Pioneer 10's arrival. One thing that's interesting is that both "experimenters" claimed to have come to a surface of crystals and volcanoes. Now we know that's nonsense, but would someone in 1973 have realized this? Was the nature of gas giants known to scientists in that era? Smurrayinchester 13:17, 10 February 2017 (UTC)
- I haven't looked too deeply into it, but using some of the filtering options in Google Scholar, I did this search using a date range likely to bring up contemporary research for your question. --Jayron32 13:23, 10 February 2017 (UTC)
- We have quite a bit about Project Stargate, Harold Sherman, Ingo Swann, we even have Russell Targ as an editor, though last I noted mostly one irate about the excessive degree to which people go to put him down in his article. I think an aspect of this to watch for though is that some (not all!) of the people involved, both as experimenters and as subjects, were members of Scientology, which had some interest in promoting its ability to create unusual powers. That said, I am partially receptive to the notion of paranormal phenomena and don't want to be too hasty to rule anything out. That said, well, "there is an enormous mountain range about 31,000 feet high..." -- where does this come from? I mean, according to the idea of remote viewing, where do you get a scale bar? Where do you get the light to see them? If it's precognition, well, it's getting pretty late for anyone to put out those measurements, and there would have been easier data to remember.
- As for what people knew, well, they knew Jupiter had a Great Red Spot, that it was an immense storm, that it was a windy planet. They also could deduce that solid material falling into Jupiter had to go somewhere. In 2001 (novel), not much later, Arthur C. Clarke talked about a diamond the size of the Earth at Jupiter's core, an idea which still has some resonance ... and still is based on a lot of guesswork. I didn't notice anything in the accounts that would have been hard to guess then and not hard to guess now, because the outer planets themselves really haven't been studied in very different ways since then (the Cassini probe has looked extensively at Saturn's rings, but if it has made any breakthroughs beyond a few features of Saturn's atmosphere I can't think of what they are) Wnt (talk) 15:47, 10 February 2017 (UTC)
- I'm not sure criticism of Targ is unjustified. One of the bedrock principles of science is Reproducibility. Together with falsifiability they are the sine qua non of science. ANYTHING which fails those two basic principles cannot be called science. In the Misplaced Pages article, criticism towards Targ's theories are based solely on the lack of ability to reproduce his results in properly controlled experiments. A pseudoscience red flag is if positive results can only be obtained by the person making the claim, and cannot be independently verified by others. --Jayron32 16:03, 10 February 2017 (UTC)
- Oh boy! What a fun question!
- I have a bunch of planetary science books from the early 1950s... and as an enthusiast of planetary science, science history, and covert activities of the Central Intelligence Agency, this question got me thinking!
- First, let's clarify a few points: CIA did actually study psychic ability for a time. Whether they ever believed in its "truth" is beside the point: in matters of intelligence, things don't have to be factually true in order to be useful.
- Now, on to the interesting planetary science: how much did we know about Jupiter in the early 1970s, prior to the Pioneer and Voyager missions?
- All my books on Jupiter are too recent to be of use here, so I had to search a wider set in my library.
- I cracked open my copy of Sagan and Shklovsky, Intelligent Life in the Universe (1966), which devotes a lot of effort to describe Jovian planets. There's no mention of a solid surface, crystals, or volcanos... they cite theorization by Soviet astrophysicists regarding a plausible biological mechanism for producing methane ("...Jovian cows..." are actually postulated by G. A. Tikhov, and he has a lunar crater named for him, so he must have some kind of credibility!) ... but Sagan and Shklovsky seem to believe in a more mundane probable cause.
- I think mainstream scientific thought, prior to the space travel era, assumed that Jovian planets would be pretty homogeneous balls of hydrogen and helium... and they were pretty close to the mark on most points, because observational astronomy provides enough data to constrain a lot of the physical parameters of planetary science. One of my books, Strange World of the Moon (Firsoff, 1962) nails precise details of geology and chemistry seven years before rock samples were acquired by American manned missions during Project Apollo. Now, if I were an intelligence agency in the 1960s and I saw such great science pouring forth from my nuclear rival, seemingly decades more advanced than our own science, I'd expend some effort to figure out every possible way they might be edging forward.
- I'll keep digging through my shelves to see if I have any older Jupiter books to compare...
- Nimur (talk) 15:52, 10 February 2017 (UTC)
- I have raked around and found my copy of The Planets by Patrick Moore, 1962 (I remember saving up and buying it as a boy). Moore describes two models and says it is not known which is correct or whether both are wrong. The earlier theory by R. Wildt (presumably Rupert Wildt) has a "rocky, metallic" core 37,000 miles in diameter surrounded by an ice layer (I think in those days "ice" in effect meant "water ice") 17,000 miles thick, and then a hydrogen-rich atmosphere 8,000 miles thick. He explains that at the base of the atmospheric layer conditions will be gaseous technically but the material will behave "in the manner of a solid". The later theory (which Moore seems to favour) is by W. Ramsay of Manchester University and proposes mainly hydrogen throughout and where it will be solid by a depth of 2,000 miles and metallic by 5,000 miles depth. Moore says "... neither can we hope that rocket probes will be able to clear this matter up in the foreseeable future, since Jupiter is so remote that for the present it is well beyond the range of even small unmanned vehicles". Thincat (talk) 17:40, 10 February 2017 (UTC)
- To go on a bit, Moore says there have been suggestions that the Great Red Spot is due to a "gigantic volcano which pokes out above the cloud layer". He completely dismisses this idea because the GRS "is known to shift some 2,000 miles to either side of its mean position". He hypothesises it may be "a solid lump 'floating' in Jupiter's atmosphere". Thincat (talk) 18:37, 10 February 2017 (UTC)
- More Moore: It is known that the different belts of Jupiter spin at different rates. "This sort of behaviour provides additional proof, if it were needed, that we are not dealing with a solid, rocky surface". (BTW he gives no suggestion that the GRS might be a vortex). Thincat (talk) 19:32, 10 February 2017 (UTC)
- Thanks Nimur and Thincat, that's really interesting stuff! Smurrayinchester 20:26, 10 February 2017 (UTC)
- The answer is that it was basically known long before that. The radius of Jupiter is easily observed, and its mass can be calculated from its gravitational pull on its satellites. This allows its density to be calculated, and from the density it is easy to infer that the great bulk of it is hydrogen. Looie496 (talk) 14:44, 12 February 2017 (UTC)
Why are my contributions not visible on the main pageJohn(Y-B-F-L) (talk)
My information is entirely factual.John(Y-B-F-L) Bates Yellow-bellied flycatcher Downy Woodpecker Hairy woodpecker — Preceding unsigned comment added by John(Y-B-F-L) Bates (talk • contribs) 15:07, 10 February 2017 (UTC)
- As you can see Here, another editor removed your changes because you did not provide a reference so other people could verify what you had written. See Misplaced Pages:Cite your sources for more information. If you need technical help with doing so, both Misplaced Pages:Help desk or Misplaced Pages:Teahouse, experienced users that frequent those desks can help you along. --Jayron32 15:13, 10 February 2017 (UTC)
- Another thing: "the main page" refers to Main page, which is currently protected to prevent vandalism. Any edits you might have attempted there would not have been saved. You can suggest changes to the main page at Talk:Main page, but please do so in a "please change X to Y because Z" format. Ian.thomson (talk) 15:19, 10 February 2017 (UTC)
- @John(Y-B-F-L) Bates: Honestly, a longer-term editor probably would have gotten away with an unsourced bit about the song - as you can tell, there are a lot of unsourced things that still exist in our articles! But when someone new shows up people tend to be more wary of mischief and confusion. Sourcing your idea is not really that easy: I found this saying that song is the best character to use, but it isn't specific. A hard thing to understand at times is that Misplaced Pages is very wary about "WP:original synthesis": for example, suppose you listen to two bird calls and see there's a four-second difference. That's great, but ... is that every bird of each type, or is the different call just from a subspecies or local population in your area? With your experience you might think of a way to find this fact -- or someone else here might -- but without a source specifically saying this is the difference, the best I could do would be to say something along the line of "The call of this bird is how to identify it. This call is available here and you can compare it to the call of that one there." And you can do that, it's valuable. You just have to build up what you can with what you have in hand. Wnt (talk) 16:59, 10 February 2017 (UTC)
- @John(Y-B-F-L) Bates: You also cannot cite yourself as a source. We don't know who you are, how knowledgeable you are, or your motives. Matt Deres (talk) 17:35, 10 February 2017 (UTC)
- To be technical, you could cite yourself, but first you'd have to get published in a source with a reliable editorial fact-checking process, per WP:RS... though even then there's potentially a whole conflict of interest thing which I don't even want to get into because its application is kind of random. Misplaced Pages has a lot of policies, you may have gathered. Wnt (talk) 11:56, 11 February 2017 (UTC)
Superglue turning white and white extending beyond superglue (on black plastic)
I had to get inside an electric transformer to fix a broken connection. The casing was glued so I cut my way in. To repair the casing, I used superglue. I've seen superglue turn white as it dries before and have had some success turning it clear again using a hair dryer (I read this was because of water absorbed by the superglue). In this case though, the white extends beyond the superglue onto the black plastic. The hair dryer made no difference to the whitening in this case. Any idea what the white is? ----Seans Potato Business 22:07, 10 February 2017 (UTC)
- Think you're observing the fumes depositing themselves. Cyanoacrylate#Forensics.--Aspro (talk) 23:15, 10 February 2017 (UTC)
- Can you make out any fingerprints ? StuRat (talk) 23:30, 10 February 2017 (UTC)
- The OP has been repairing it. Any prints would be smudged beyond recognition.--Aspro (talk) 23:41, 10 February 2017 (UTC)
- Not necessarily. It depends on if the last few touches left a good fingerprints. StuRat (talk) 02:24, 11 February 2017 (UTC)
- I don't think it is this but worth a check - could the plastic have been stressed turning it white? Or are you sure it is a surface effect? Dmcq (talk) 11:34, 11 February 2017 (UTC)
- I've had the fingerprint effect when I've been using cyanoacrylate, but you could get the same effect on any stress ridges. I suppose it could also be a reaction with the old glue. You could try cleaning it off with acetone, though that might dissolve the plastic too. Cyanoacrylate marks can sometimes be removed with water and light abrasion (it's probably the abrasion more than the water because its solubility in water is extremely slow). Vegetable oil or petroleum jelly might work better to remove or hide the white marks. Dbfirs 12:09, 11 February 2017 (UTC)
- I believe (!) that when you broke the casing you created a set of stress cracks that lead away from the cut. as the Cyanoacrylate paste disspersed into these cracks it might have dissolved some of the polymer casing and the resultant patterns were made. the white is usually an indication of inclarities due to dissolved water but more likely are a result of crystals formed in the glue as it dried over the polymer. 109.67.54.194 (talk) 17:05, 12 February 2017 (UTC)
- As far as how to improve the aesthetics, I suggest you wrap black electrical tape around it, unless it has a problem with getting hot, which that could make worse. StuRat (talk) 17:48, 12 February 2017 (UTC)
February 11
Why people with hypoxemia get blue color (cyanosis) on their skin?
Why people with hypoxemia get blue color (cyanosis) on their skin? Iv'e done googling but I got confusing answers. One article claims that it's because of the deoxyhemoglobin and another one claims that it's not the reason. In fact, I would like to know a simple answer for why deoxigenation causes to appearance of bluish skin in a way that it looks like as if the blood is blue (obviously, I absolutely know that the blood is not really blue but I do understand generally that there is an issue of illusion by the wavelengths and light absorption. It's not clear to me especially with thr relation to the ). 93.126.88.30 (talk) 00:11, 11 February 2017 (UTC)
- The two articles you link to are about different phenomena. The first is about cyanosis (bluish skin), the second is about why veins seem blue - a related but not identical situation. I think you'll find the deoxyhemoglobin explanation is the usual explanation for cyanosis, though you may find other viewpoints. You can find various articles that are somewhat pertinent by googling the phrase "Mechanism of cyanosis". (rather than "cause of cyanosis, which leads to differential diagnoses). Since there are different causes of cyanosis working via different mechanisms, no explanation is going to be simple. - Nunh-huh 00:58, 11 February 2017 (UTC)
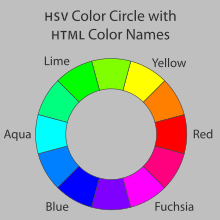
- Well oxygenated blood is bright red, while poorly oxygenated blood is rather dark. If you consider that red and cyan are on opposite sides of the color wheel, a lack of red where expected would cause a relatively darker or cyanic look. Of course there are all sorts of color wheels, and different types of pigmentation, in case anyone wants to nitpick. But the RGB color wheel is quite pretty. Exposing old bluish sealed ground beef to the air will turn it red again if it has not gone totally off. μηδείς (talk) 03:53, 11 February 2017 (UTC)
Aircraft tendency to roll?
In an episode of Mayday, it was mentioned that the accident aircraft in that episode had a natural tendency to roll to the right, and that it was typical for older aircraft (this particular aircraft was 17-18 years old at the time of the accident) not to fly level by default. Do aircraft acquire a natural tendency to roll to one side or another over time, or is this tendency present from the moment they enter into service? 173.52.236.173 (talk) 04:53, 11 February 2017 (UTC)
- I've never heard of that, and can't find anything from quick searching. Do you know which episode it was in? Someguy1221 (talk) 05:04, 11 February 2017 (UTC)
- The subject accident is Adam Air Flight 574. 173.52.236.173 (talk) 05:19, 11 February 2017 (UTC)
- Our article mentions an uncorrected roll, but nothing about the aircraft's age causing it. The rudders on many 737s had a tendency to turn on their own, inducing both a yaw and a roll , so it might have been true in the sense that "737s built in this particular time window have a tendency to roll". No idea really if that's what the people in that episode were talking about. Someguy1221 (talk) 05:30, 11 February 2017 (UTC)
- Among the many non-ideal effects that can cause uncommanded roll, p-factor is the first that comes to my mind... (it's more of an uncommanded yaw than a roll); but I'll have to take a closer look at the source material to see whether this specific effect is relevant to the example in this question. Nimur (talk) 08:02, 11 February 2017 (UTC)
- Not relevant to the example in question -- jets don't have P-factor. 2601:646:8E01:7E0B:F88D:DE34:7772:8E5B (talk) 10:14, 11 February 2017 (UTC)
- Indeed, very true - I ought not even have mentioned that, as p-factor is not specifically relevant here! I apologize for jumping in with an answer before fully briefing myself on the information pertinent to this question.
- It looks like the aircraft in the OP's event was a 737. If you direct your search for the terms "737 uncommanded roll" you'll find a lot of coverage, e.g. this 1996 article in Aviation Week: NTSB Probes 737 Uncommanded Roll. That event was related to the yaw damper. In layman's terms, a yaw damper is sort of like a part of the "autopilot" that is meant to help keep the airplane stable; but it is in fact a separate "line replaceable unit" - a separate subsystem - that can drive the control surfaces of the very complicated aircraft. In the FAA's detailed supplemental report on USAir Flight 427, there is a lot of discussion about how an engineering malfunction and a defective design may have contributed to uncommanded - or even reverse-commanded - flight control deflection, in early versions of the Boeing 737.
- Nimur (talk) 17:31, 11 February 2017 (UTC)
- At least some Boeing 737's used turbofan engines. Would that introduce gyroscopic effects ? If so, that would tend to resist any change in direction, although presumably not enough to be a problem, or they wouldn't use that design. StuRat (talk) 00:53, 12 February 2017 (UTC)
Why do boys like girls long before spermarche?
Why would evolution favor that? This seems the complete opposite of the male evolutionary motif, shouldn't the average age of spermarche be 10? Why make us want girls at an age when they'll be absolutely no reproducing? Sagittarian Milky Way (talk) 14:23, 11 February 2017 (UTC)
- I don't think you could say that evolution favors that. The mechanisms of sexual attraction are probably in place separately from the ability to ejaculate, and timewise they approximate one another. If perhaps sexual attraction precedes the capacity to produce offspring, there doesn't seem to me to be any great cost associated with that amorousness. Bus stop (talk) 14:41, 11 February 2017 (UTC)
- Who says they do? ←Baseball Bugs carrots→ 15:04, 11 February 2017 (UTC)
- Spermarche says 11-15 for an average of 13. Sagittarian Milky Way (talk) 15:16, 11 February 2017 (UTC)
- BB's question is legitimate - at what age do boys typically start feeling sexual attraction towards females? Personally, I didn't really experience it until the beginning of puberty (when testosterone levels begin to rise), but that's only anecdotal. Does the research support the OP's contention, that male attraction to females typically pre-dates spermarche? Eliyohub (talk) 15:58, 11 February 2017 (UTC)
- The gap was almost 4 years for me. That's like half of adolescence. Sagittarian Milky Way (talk) 16:26, 11 February 2017 (UTC)
- Even if it did, why should we find this anomalous? Is there an outsized biological cost to sexual attraction preceding actual ability to reproduce? If so, what would it be? I don't think there is any cost, but maybe I'm overlooking something. Bus stop (talk) 16:14, 11 February 2017 (UTC)
- I'm not sure how good it is but this source suggests around 9-10 is common (it includes a ref) and it appears to
coincide withoccurs after adrenarche (also includes a ref) . Edit I should have said occurs after adrenarche which is what the book says, now corrected. As far as I can see it's not implying there's necessarily a connection, it only deals a small amount with the science anyway. I'd note from the time frames mentioned for adrenarche, it may be at least 1 year and maybe up to 3 after depending on various things including I suspect how exactly you define the start. Nil Einne (talk) 03:16, 13 February 2017 (UTC)
- I'm not sure how good it is but this source suggests around 9-10 is common (it includes a ref) and it appears to
- Note that children practice a wide range of adult behavior, such as playing with plastic tools and changing diapers on dolls. In this context, it's just one more thing to practice. Also, since humans are social animals, establishing relationships with the opposite sex early on might help to maintain those relationships until after puberty. StuRat (talk) 16:44, 11 February 2017 (UTC)
- Perhaps this is a preparatory behaviour. Many predatory non-human animals perform substantial amounts of play behaviour which is considered to be preparation for hunting and catching prey when they are older. This happens at an age when they are still getting all their food requirements from their mum and/or dad. DrChrissy 17:05, 11 February 2017 (UTC)
- Following on from SR & DrChrissy. Think it is simple. Evolution encouraged our ancient ancestors to start looking for suitable mates and establish a strong a bond before breeding begins. Even animals exhibit and practice courtship behaviour as juveniles. Playing with dolls is a cultural thing (which is learnt). Getting interested in the opposite sex as possible future dependable partner is more instinctive driven. Boys are 'instinctively' attracted to girls with good figures because it is advertising that she is in good breeding condition and girls are 'instinctive' attracted to boys that demonstrate (through actions and behaviour) that they will be able to provide and bring home the bacon, put bread on the table to feed the family and protect them from harm. Behaviour after that bond is created becomes X rated so can't comment.--Aspro (talk) 17:26, 11 February 2017 (UTC)
- Oh Gosh. Hope I haven't taken the love & romance out of all this! Use common sense by all-means but follow your instincts and bond. Beware of one's closest friends and confidants – it is also in their selfish genes to 'subconsciously' bugger a relationship up for you with their well-meant but stupid advice. This is especially important if having attended a single gender school. Ones own sex can be your worst enemies when it comes to love and romance (both of which should be and remain private).--Aspro (talk) 17:55, 11 February 2017 (UTC)
- If the sperm production preceded the amorous attraction one could argue that there is a biological cost in sperm production. But as it is the other way around the question is what cost is there in amorous attraction? Bus stop (talk) 17:38, 11 February 2017 (UTC)
- Can't stop thinking about her, fail to notice a lion and get eaten, falling in love takes an inordinate amount of time away from learning hunting, will before a way decreases the average number of STD-less offspring... Sagittarian Milky Way (talk) 18:26, 11 February 2017 (UTC)
- For a start there is the cost of diapers , medical bills, lawyers fees to get the teenage father to accept some responsibility. Possible failure for the young mother to complete her high school education... The list of costs go on and on. In more the more primitive societies of ancient ancestors it was a death sentence for the new born. --Aspro (talk) 18:16, 11 February 2017 (UTC)
- I think we might be considering this in a too-modern human perspective. Back in the good old days, I think if I had been 10-13 and fancying a a young Elle MacPherson, I would have still run away like stuff off a chrome shovel if I saw an approaching lion. Who knows, I might have pushed Ell in the way of the lion to increase my chances of surviving and subsequently mating! Our motivations are quite primeval!DrChrissy 18:57, 11 February 2017 (UTC)
- Did people that age in lion country usually carry an anti-lion device? (pointy stick?) Sagittarian Milky Way (talk) 19:13, 11 February 2017 (UTC)
- I would imagine so, and very probably a couple of rocks and perhaps a slingshot-type device - but the Fight-or-flight response would have kicked in and throwing Elle to the lion and running like hell might have been the behavioural final common path. DrChrissy 20:26, 11 February 2017 (UTC)
- Amorous feelings do not impact upon the chances of survival or eventual reproduction. The question asks why would evolution favor boys liking girls before spermarche? Evolution does not favor boys liking girls before spermarche. The observed phenomenon is irrelevant to evolution. Unless of course someone can actually name a consequence, such as a disadvantage in survival or eventual reproduction, associated with early-onset amorousness. Bus stop (talk) 20:41, 11 February 2017 (UTC)
- But I have suggested a potential advantage (preparation for adult life) and Sagittarian Milky Way has suggested potential disadvantages (getting distracted and thereby eaten by a lion). Admittedly, these are both OR, but they are both plausible. Note that the cost of having a motivation must be incredibly small - it is the behaviour of acting upon the motivation which is likely to have an effect on fitness. DrChrissy 20:49, 11 February 2017 (UTC)
- Hence the observed phenomenon is irrelevant to evolution, because an advantage and a disadvantage would cancel one another out. Bus stop (talk) 21:36, 11 February 2017 (UTC)
- Absolutely not - it would depend on the relative strengths of the advantages and disadvantages to fitness. DrChrissy 21:50, 11 February 2017 (UTC)
- Both of which you concede are original research. Bus stop (talk) 22:00, 11 February 2017 (UTC)
- Yes, I stated that - did you read my post? DrChrissy 22:31, 11 February 2017 (UTC)
- In my opinion there is a distinct difference between something physical (the development of gametes) and something behavioral (sexual attraction). Evolution has to carefully allocate precious biological resources for one purpose or another. But evolution can afford to play much more fast and loose with mere behavior that in any case doesn't lead to pregnancy hence the passing on of traits. Bus stop (talk) 03:44, 12 February 2017 (UTC)
- You are speaking as if you believe evolution has a master-plan or a template it is working toward. This is not the case. Evolution is what we see as the result of random mutations (which affect both behavioural and physiological attribute). Furthermore, evolution has not finished...it is an ongoing process. DrChrissy 18:24, 12 February 2017 (UTC)
- What evidences that I am "speaking as if believe evolution has a master-plan or a template it is working toward"? Is there something I've said that suggests this? Bus stop (talk) 19:52, 12 February 2017 (UTC)
- Your sentence "Evolution has to carefully allocate precious biological resources for one purpose or another." DrChrissy 19:57, 12 February 2017 (UTC)
- A living organism has many competing demands. Developing viable gametes is just one of those many competing demands. Many systems are developing during a time of prepubescence. Assuming that sexual attraction precedes by a few years the development of viable gametes, this is somewhat inconsequential in determining the course evolution takes. Biological assets diverted to gamete production are not available for other pressing needs. But the mere attraction for the opposite gender in the absence of any ability to actually reproduce does not factor into evolutionary pressures. For one thing, behavior does not in any obvious way detract from the body's various developing systems. But of additional importance is that such behavior does not pass on genetic material due to the fact that pregnancy does not take place. Bus stop (talk) 20:30, 12 February 2017 (UTC)
- What you have written is OR. I say that not in a derogatory way, but recognising it for what it is...because I am also about to write OR. You stated
But the mere attraction for the opposite gender in the absence of any ability to actually reproduce does not factor into evolutionary pressures.
What about this consideration. What if my becoming attracted to girls at a younger age makes me interact with them more at that age, and then when I become sexually mature I am more able to attract mates as a consequence of this prepubescent motivation and behaviour. That certainly would factor into evolutionary pressures. DrChrissy 20:48, 12 February 2017 (UTC)
- What you have written is OR. I say that not in a derogatory way, but recognising it for what it is...because I am also about to write OR. You stated
- A living organism has many competing demands. Developing viable gametes is just one of those many competing demands. Many systems are developing during a time of prepubescence. Assuming that sexual attraction precedes by a few years the development of viable gametes, this is somewhat inconsequential in determining the course evolution takes. Biological assets diverted to gamete production are not available for other pressing needs. But the mere attraction for the opposite gender in the absence of any ability to actually reproduce does not factor into evolutionary pressures. For one thing, behavior does not in any obvious way detract from the body's various developing systems. But of additional importance is that such behavior does not pass on genetic material due to the fact that pregnancy does not take place. Bus stop (talk) 20:30, 12 February 2017 (UTC)
- Your sentence "Evolution has to carefully allocate precious biological resources for one purpose or another." DrChrissy 19:57, 12 February 2017 (UTC)
- What evidences that I am "speaking as if believe evolution has a master-plan or a template it is working toward"? Is there something I've said that suggests this? Bus stop (talk) 19:52, 12 February 2017 (UTC)
- You are speaking as if you believe evolution has a master-plan or a template it is working toward. This is not the case. Evolution is what we see as the result of random mutations (which affect both behavioural and physiological attribute). Furthermore, evolution has not finished...it is an ongoing process. DrChrissy 18:24, 12 February 2017 (UTC)
- In my opinion there is a distinct difference between something physical (the development of gametes) and something behavioral (sexual attraction). Evolution has to carefully allocate precious biological resources for one purpose or another. But evolution can afford to play much more fast and loose with mere behavior that in any case doesn't lead to pregnancy hence the passing on of traits. Bus stop (talk) 03:44, 12 February 2017 (UTC)
- Absolutely not - it would depend on the relative strengths of the advantages and disadvantages to fitness. DrChrissy 21:50, 11 February 2017 (UTC)
- Hence the observed phenomenon is irrelevant to evolution, because an advantage and a disadvantage would cancel one another out. Bus stop (talk) 21:36, 11 February 2017 (UTC)
- But I have suggested a potential advantage (preparation for adult life) and Sagittarian Milky Way has suggested potential disadvantages (getting distracted and thereby eaten by a lion). Admittedly, these are both OR, but they are both plausible. Note that the cost of having a motivation must be incredibly small - it is the behaviour of acting upon the motivation which is likely to have an effect on fitness. DrChrissy 20:49, 11 February 2017 (UTC)
- Amorous feelings do not impact upon the chances of survival or eventual reproduction. The question asks why would evolution favor boys liking girls before spermarche? Evolution does not favor boys liking girls before spermarche. The observed phenomenon is irrelevant to evolution. Unless of course someone can actually name a consequence, such as a disadvantage in survival or eventual reproduction, associated with early-onset amorousness. Bus stop (talk) 20:41, 11 February 2017 (UTC)
- I would imagine so, and very probably a couple of rocks and perhaps a slingshot-type device - but the Fight-or-flight response would have kicked in and throwing Elle to the lion and running like hell might have been the behavioural final common path. DrChrissy 20:26, 11 February 2017 (UTC)
- Did people that age in lion country usually carry an anti-lion device? (pointy stick?) Sagittarian Milky Way (talk) 19:13, 11 February 2017 (UTC)
- I think we might be considering this in a too-modern human perspective. Back in the good old days, I think if I had been 10-13 and fancying a a young Elle MacPherson, I would have still run away like stuff off a chrome shovel if I saw an approaching lion. Who knows, I might have pushed Ell in the way of the lion to increase my chances of surviving and subsequently mating! Our motivations are quite primeval!DrChrissy 18:57, 11 February 2017 (UTC)
- For a start there is the cost of diapers , medical bills, lawyers fees to get the teenage father to accept some responsibility. Possible failure for the young mother to complete her high school education... The list of costs go on and on. In more the more primitive societies of ancient ancestors it was a death sentence for the new born. --Aspro (talk) 18:16, 11 February 2017 (UTC)
- One possible evolutionary pressure against this is if it spreads diseases, like mononucleosis and oral herpes, which decrease the chances of passing on your genes. StuRat (talk) 21:06, 12 February 2017 (UTC)
I was actually thinking of non-sexual interactions such as learning courtship behaviours not leading to mating, but you are correct if sexual interaction occurs. DrChrissy 21:14, 12 February 2017 (UTC)
- You can spin any tale. You can argue that prepubescent sexual behavior favors or disadvantages the later passing on of genetic material. But you cannot argue, and you have not argued, that the development of viable gametes does not figure prominently in the evolutionary directions that the species takes. What we know is that the point in time that the ability to reproduce has a clear and direct influence on the evolutionary directions that the species takes. But you cannot make the same argument with nearly the same degree of assuredness that the display of non-viable (for reproduction) sexuality figures into the direction that evolution takes the species with each succeeding generation. One thing is known, the other thing is merely hypothesized about. It is not a matter of whether the "research" is "original" or not. The realm of the physical is different from the realm of the behavioral. Materials are costly. Behavior may or may not provide an evolutionary pressure. The particular behavior under consideration does not, in and of itself, result in the passing on, of genetic material. Thus you have to spin a tall tale to argue that prepubescent sexual attraction plays a part in the direction taken in the evolution of the species. Bus stop (talk) 21:19, 12 February 2017 (UTC)
- Wow! This is beginning to feel like an attacking rant. I have been honest and open where I have posted OR mainly because I am very mindful this is a reference desk, so your calling these "tall tales" is rather objectionable. They are scientifically plausible ideas. Early experience in animals is extremely important for the successful development of even some of the most basic behaviours. For example, if commercial chickens are raised without perches, they have to "learn" how to use perches in subsequent housing where perches are present (yes, I can provide the research articles). As for your comment "Behavior may or may not provide an evolutionary pressure." I do not see why you are limiting this to behaviour when the same is true of physiology - I suggest you read Vestigiality and then ask yourself why do our mammalian bodies use resources to produce nipples on the males when these have no function? Why do some species of cave fish develop eyes only to have these become covered in skin? Physiology is no better than behaviour at reacting to evolutionary pressures. But overall, it feels like you are moving the goalposts in this discussion and I have no idea why. DrChrissy 22:07, 12 February 2017 (UTC)
- The question asks "Why do boys like girls long before spermarche? Why would evolution favor that?" The answer is that evolution does not favor that. That particular behavior has no known pressure on evolution. Liking girls in the absence of the ability to pass on genetic material fails to create a pressure on evolution. Bus stop (talk) 22:19, 12 February 2017 (UTC)
- Thanks for completely ignoring my post. OK - let's get to the nitty gritty. Where is the evidence for what you are stating? Let's remember "Absence of evidence is not evidence of absence". DrChrissy 22:25, 12 February 2017 (UTC)
- Evolution doesn't "favor" all things. Some observable phenomena are of secondary importance. Spermarche has to be "favored" by evolution due to its central role in reproduction. But "liking girls" (before spermarche) may not be the result of natural selection. It doesn't have such a central role in reproduction. (The question asked was "Why do boys like girls long before spermarche? Why would evolution favor that?") Bus stop (talk) 06:52, 13 February 2017 (UTC)
- The new born of higher primates take longer to develop and become independent. During that time they not only need feeding but protection from other members of the troop that would cause them harm and even kill them – and then want to mate with the female- so that his selfish gene gets passed on. Therefore, it is important for the female to bond with a strong male of the troop to ensure the survival of her offspring. Remember too, that in humans, gestation is nine months and that (biologically) is quite an investment for an female animal. The male instinct evolved to compliment. Modern day 'civilized' humans still exhibit this type of behaviour in war situations – we are still animals in that respect. This is nature both red in tooth and claw and we can't separate ourselfs from that. So this is the benefit of bonding before Spermarche. Hence, boys get interest in girls at an early age and girls get interest in boys... --Aspro (talk) 22:00, 11 February 2017 (UTC)
- Evolution doesn't "favor" all things. Some observable phenomena are of secondary importance. Spermarche has to be "favored" by evolution due to its central role in reproduction. But "liking girls" (before spermarche) may not be the result of natural selection. It doesn't have such a central role in reproduction. (The question asked was "Why do boys like girls long before spermarche? Why would evolution favor that?") Bus stop (talk) 06:52, 13 February 2017 (UTC)
- Thanks for completely ignoring my post. OK - let's get to the nitty gritty. Where is the evidence for what you are stating? Let's remember "Absence of evidence is not evidence of absence". DrChrissy 22:25, 12 February 2017 (UTC)
- The question asks "Why do boys like girls long before spermarche? Why would evolution favor that?" The answer is that evolution does not favor that. That particular behavior has no known pressure on evolution. Liking girls in the absence of the ability to pass on genetic material fails to create a pressure on evolution. Bus stop (talk) 22:19, 12 February 2017 (UTC)
- Wow! This is beginning to feel like an attacking rant. I have been honest and open where I have posted OR mainly because I am very mindful this is a reference desk, so your calling these "tall tales" is rather objectionable. They are scientifically plausible ideas. Early experience in animals is extremely important for the successful development of even some of the most basic behaviours. For example, if commercial chickens are raised without perches, they have to "learn" how to use perches in subsequent housing where perches are present (yes, I can provide the research articles). As for your comment "Behavior may or may not provide an evolutionary pressure." I do not see why you are limiting this to behaviour when the same is true of physiology - I suggest you read Vestigiality and then ask yourself why do our mammalian bodies use resources to produce nipples on the males when these have no function? Why do some species of cave fish develop eyes only to have these become covered in skin? Physiology is no better than behaviour at reacting to evolutionary pressures. But overall, it feels like you are moving the goalposts in this discussion and I have no idea why. DrChrissy 22:07, 12 February 2017 (UTC)
Small female dogs getting knocked up by big male dogs
Sorry for the seemingly pornographic title, this is a serious and legitimate question.
If, for example, a male Great Dane (the biggest dog breed) managed to mate with and impregnate a female Chihuahua (dog) (the smallest dog breed - believe me, when there's a will, there's a way!), would there be a risk of the puppies being so big in utero (during the pregnancy), such that they would pose a risk to the Chihuahua mother? Or has nature already taken this into consideration, and the unborn puppies would still be small enough to make the pregnancy no more risky than any other?
I've encountered a dog whose dad was a Jack Russell and whose mom was a Border Collie (yes, they somehow managed to mate!), but never the sort of reverse situation (where the father dog was a far bigger breed than the mother). Anyone able to answer my question? I've long pondered this. Eliyohub (talk) 15:55, 11 February 2017 (UTC)
- Not an answer to the question, but an analogy: a hinny has a horse father and a (generally smaller) donkey mother (the opposite of a mule). Our article says "Hinnies on average are slightly smaller than mules in part because donkeys are generally smaller than horses, and growth potential of equine offspring is influenced by the size of the dam's womb". This supports the notion that womb size can affect offspring size while allowing for viability. Whether this works in extreme cases like a giant male dog and a tiny female dog is another question. Is there a cutoff point for relative sizes for viability? Loraof (talk) 17:52, 11 February 2017 (UTC)
- This question has come up several times previously on the ref desks, as here. I don't recall that a definitive answer has ever been given, though. Deor (talk) 18:25, 11 February 2017 (UTC)
- My own experience (limited, but perhaps more than most as I worked as a vet nurse) of newborn pups is that their size is relatively constant. An adult great dane which might weigh 10 x (my figure) an adult chihuahua does not give birth to pups that are 10 x larger. This graph here seems to support this. there is also this Dog development compensates for this by larger dogs taking longer to develop and stop growing. DrChrissy 19:10, 11 February 2017 (UTC)
- The question is intriguing; I'm not sure how to answer it but I went searching for in vitro fertilization in dogs, and found it only recently has been done. The Newsweek article says that the bitch was a hound, chosen because it is larger then the cocker spaniel x beagle or beagle x beagle pups. I see nothing in the article about whether the pups turned out larger because of the larger womb size, but potentially this would be a great way to look for influence because you might use highly inbred animals and look for non-genetic differences from in different bitches. Wnt (talk) 18:12, 12 February 2017 (UTC)
Humidity in heated room vs humidity in cold air
If someone is in a heated room and closed room, would he reduce or increase the humidity by aerating the room regularly? Would this change the thermal sensation?--Terurme (talk) 18:32, 11 February 2017 (UTC)
- By "aerating" do you mean opening windows? ←Baseball Bugs carrots→ 19:12, 11 February 2017 (UTC)
- Yes, maybe for a couple of minutes each hour.--Terurme (talk) 19:18, 11 February 2017 (UTC)
- Assuming by "humidity" you mean relative humidity it will depend on the humidity outdoors, the humidity indoors, the temperature outdoors, and the temperature indoors. Shock Brigade Harvester Boris (talk) 19:24, 11 February 2017 (UTC)
- We can assume that the temperature indoors is higher than outside, that's why the room is being heated. We can also assume that the humidity was the same at the beginning. --Terurme (talk) 19:39, 11 February 2017 (UTC)
- If there are people in the room, or cooking going on then the absolute humidity in the closed room should increase. Swapping that air and reheating will decrease the humidity. Graeme Bartlett (talk) 20:22, 11 February 2017 (UTC)
- Taking User:Terurme's conditions of identical starting relative humidity indoors and outdoors we can specify two possible outcomes:
- If the outdoor air is brought in and heated to the original indoor temperature, the temperature will be the same as the original indoor temperature (because we've made it so) and relative humidity will decrease.
- If the outdoor air is brought in and mixed with the indoor air without heating, the temperature will be somewhere between the original outdoor and indoor temperatures and the indoor relative humidity will increase.
- Should there be sources or sinks of moisture within the room (as Graeme mentions) those will alter the indoor humidity correspondingly. Shock Brigade Harvester Boris (talk) 21:37, 11 February 2017 (UTC)
- Taking User:Terurme's conditions of identical starting relative humidity indoors and outdoors we can specify two possible outcomes:
- If there are people in the room, or cooking going on then the absolute humidity in the closed room should increase. Swapping that air and reheating will decrease the humidity. Graeme Bartlett (talk) 20:22, 11 February 2017 (UTC)
- We can assume that the temperature indoors is higher than outside, that's why the room is being heated. We can also assume that the humidity was the same at the beginning. --Terurme (talk) 19:39, 11 February 2017 (UTC)
- Assuming by "humidity" you mean relative humidity it will depend on the humidity outdoors, the humidity indoors, the temperature outdoors, and the temperature indoors. Shock Brigade Harvester Boris (talk) 19:24, 11 February 2017 (UTC)
- Yes, maybe for a couple of minutes each hour.--Terurme (talk) 19:18, 11 February 2017 (UTC)
- Note that some forms of heating directly increase humidity. In third world nations, an open fire in the center of a yurt or other structure, with a hole in the roof for ventilation, is often used. And see kerosene_heater#Moisture_Problems (propane heaters or heating a home with a gas stove/oven have a similar issue). Also, a "leaky" radiator system may release steam into the room. So, with such a system, opening a window to let out humidity would be counter-productive, as replacing the heat would also replace the humidity. StuRat (talk) 01:09, 12 February 2017 (UTC)
- So wait, are you wanting to reduce the humidity, when it's cold? That strikes me as unusual. One of the problems with heating is very low relative humidity (because cold air with low absolute humidity, even if it's saturated with water vapor in absolute terms) is being warmed. Leads to dry skin and such. --Trovatore (talk) 05:19, 12 February 2017 (UTC)
- One reason to reduce humidity indoors in winter is that it can otherwise condense on cold windows and exterior walls and cause mold to grow. Better insulation is another option, but not always possible to add to older homes. StuRat (talk) 05:24, 12 February 2017 (UTC)
- Dampness in homes during winter is a common problem in a fair bit of New Zealand, partially because winters in some parts of NZ (Auckland for example) are quite wet. As this source mentions, it should be managable but a combination of inadequate heating (most homes aren't centrally heated), inadequate ventilation and inadequate insulation means it persists. These can be related and can conflict. Inadequate insulation obviously means what heating there is is far less effective. On the flipside, as that source and this source mentions, improved insulation can increase damp problems due to the reduce airflow/"breathing" of the house. Even worse if clothes are dried inside or a clothes dryer vented inside is used. (Other things like the lack of a range hood probably don't help either. This government advice should give an idea of the various possible problem sources.)
- If someone isn't able to improve things properly for whatever reason, using a dehumidifier to help reduce dampness isn't uncommon during winter for those who can afford it (various source mention this). (These also have the advantage of heating the room slightly, and with a generally higher COP than simple resistive heating.) As these sources mention it isn't just something Kiwis notice but many immigrants from temperate areas too.
- In terms of the OPs question, with the NZ situation if you lack adequate ventilation simply opening your windows for a time during the day particularly when it's sunny, is a common recommendation . Also opening windows if you lack adequate extraction while cooking, showering etc . There are obviously going to be situations where it makes it worse.
- Nil Einne (talk) 14:36, 12 February 2017 (UTC)
- Also note that in some places it may be possible to open windows to let the humidity out without letting the heat out. Say the temp drops to the 40's F at night, but rises to the 70's F during the day. You might have the heat on at night, but then be able to air the place out in the day. StuRat (talk) 15:01, 12 February 2017 (UTC)
Making breathable oxygen from water
So water is H2O which only has one oxygen atom in it, so it's O1. If you perform electrolysis on water, you'll get O1 and not O2. I was under the impression that O1 is toxic to breath in. Does O1 automatically form O2 after electrolysis? ScienceApe (talk) 19:49, 11 February 2017 (UTC)
- Yes, it forms molecule automatically. Ruslik_Zero 19:53, 11 February 2017 (UTC)
- See monatomic oxygen for a little more information. It's pretty sparse, though; someone should flesh it out. Basically it's a free radical and has a very strong tendency to combine, either with other oxygen atoms or with whatever's handy. Not much seems likely to make it to your lungs. I don't know how much ozone might come out of the reaction, though. That strikes me as potentially more of a problem. --Trovatore (talk) 19:56, 11 February 2017 (UTC)
- Don't know if you have ever heard of a web site called Misplaced Pages but it has an article about almost everything. Electrolysis_of_water#Equations--Aspro (talk) 20:12, 11 February 2017 (UTC)
- Misplaced Pages even has an page about wikipedia having an article about almost everything: WP:WHAAOE. DMacks (talk) 20:21, 11 February 2017 (UTC)
- The chemical formula for the electrolysis of water reaction is 2H2O + energy → 2H2 + O2. StuRat (talk) 20:37, 11 February 2017 (UTC)
- @DMacks: Best to leave out the snark, because Misplaced Pages leaves a lot to be desired here. See . According to that, the chemical mechanism is still a bit unsure. But it seems to be something where water becomes coordinated to a metal surface, such as Pt, then splits in a process (like oxidative addition?) where it has -OH tacked on to one atom and -H tacked onto the other. (is forming Pt-H "reductive addition"? H has electronegativity 2.20 and Pt 2.28, according to our article) Anyway, once some water is split, the H can join up with another and become H3O+, and the OH can become OH-, with an electron from the current source. Lone O attached to Pt or other metal would be relatively high energy if released, so unless there's a large overpotential I'm thinking it won't be, but will have to wander around on the surface until it finds a partner? Wnt (talk) 20:58, 11 February 2017 (UTC)
- Aren't you talking about Aspro's snark, not mine? I didn't comment on the mechanism because I don't know what it is (not my area of chemistry) and conversely that I often hear it's still an active area of research. Google scholar has almost 1500 hits just for mechanism of electrolysis of water in 2017 already. DMacks (talk) 21:17, 11 February 2017 (UTC)
- !Yes, you're right - I lost his signature in the links, sorry! Wnt (talk) 22:22, 11 February 2017 (UTC)
- Think in that case, Wnt should up date the WP article as he pontificates to having a deeper understanding!--Aspro (talk) 22:19, 11 February 2017 (UTC)
- Well, I can't include any question marks in the article... ought to do something, though, if someone else doesn't do it better first. Wnt (talk) 22:23, 11 February 2017 (UTC)
- Aren't you talking about Aspro's snark, not mine? I didn't comment on the mechanism because I don't know what it is (not my area of chemistry) and conversely that I often hear it's still an active area of research. Google scholar has almost 1500 hits just for mechanism of electrolysis of water in 2017 already. DMacks (talk) 21:17, 11 February 2017 (UTC)
February 12
Thanks for your information on the loudness the flying insects but i have still a question that has been unanswered
I have seen a youtube video that is deletet now. there where people trying to catch a oliathus regius female that was taking of later high in the trees. Do you think they could actually hear it flying away? Saludacymbals (talk) 16:04, 12 February 2017 (UTC)
- If you mean Goliathus regius which is a large beetle, it's possible. Here's a video about breeding them. Blooteuth (talk) 19:41, 12 February 2017 (UTC)
Antimatter
| long confused/pseudoscientific rant by single purpose account, no req for refs |
|---|
| The following discussion has been closed. Please do not modify it. |
|
wiki refers to matter and antimatter, and thus when the two are put together merged if you will, the result is essentially nothing, to me this is illogical because if matter is mass/energy and thus something how can less than nothing "antimatter" be possible? I can agree that particle may have negative charge via a pseudo zero quanta Eg such as it is with a centre tapped transformer feeding an asymmetrical power supply, having the centre tap as the pseudo zero and thus a relative positive and a relative negative voltage, "but note that zero is in fact a positive quanta" Thus much the same construct is possible with particles charges quanta, But how can "something" anything be merged with less than "nothing" to which results with nothing? can any one see how asinine that is? in short matter and less than zero matter or antimatter is simply asinine. Also when it comes to the Universe I want to know why it is not treated as a finite seeing it adheres to conservation? Eg: something can only begot from something else and nothing can never EVER contradict and be something, this means the universe is a finite quanta that has always existed, a finite because if we refer to everything then nothing is excluded and thus we indeed can rely on conservation to that finite quanta "100% or check sum to everything equating to 1 and thus all parts "constituents" are a percentage of it", meaning expansion, mind you a perceived accelerating expansion must be at the expense of some other unaccounted quanta, such as each and every Galaxy what with its core being a super black hole. Having said that though, I am quite sure, this accelerating expansion will be debunked, debunked once relativity data has been properly considered, data that has galaxies with an inward momentum, due to each and every stars storing energy via fusion, Eg: Merging two lighter atoms into a single heavier atom, thus increasing near vacuums "or an area with little mass that is mostly energetic" expansion, and thus taking up an atoms space for every fusion, to which as said near vacuum expands inwards towards new heavier atom said atoms gravity increases, to which if we apply that to the entire galaxy, what we indeed have is a perceived accelerating expansion specially if we rely on red shift changes..Korallrbare (talk) 21:16, 12 February 2017 (UTC)
|
- When matter and antimatter merge, the result isn't nothing, it's a great deal of energy, described by E = mc. See mass-energy equivalence. StuRat (talk) 21:59, 12 February 2017 (UTC)
- I agree the OP is confused / confusing / over my head. But the modern theory of antimatter arose from the Dirac sea, the antielectron / positron thought of as a "hole in the sea of negative-energy electrons". Perhaps the OP had been reading about that.John Z (talk) 23:04, 12 February 2017 (UTC)
- In addition to what StuRat mentioned, as our article says, antimatter is also mass/energy. The mass of corresponding antimatter is the same as the mass of matter. In that manner, it's misleading to call it less than nothing. If you call matter something, you could say I guess that it's an opposite form of something. Note the key word "form" here. I didn't say the opposite of something but the opposite form of something. Antimatter is still something, it's just a different, opposite type of something. (Incidentally any confusion here is why you should take care when trying to make English words correspond to concepts.) Consider what this means. As a being made of matter, I couldn't hold a ball of antimatter without what StuRat mentioned happening. But if I was a being of antimatter, a ball of antimatter would seem exactly the same as a corresponding ball of matter seems to me the being of matter. Of course if I were antimatter, unless I was magically converted from matter or something like that, I'd just call it matter and the other stuff would be antimatter. (Baryon asymmetry does complicate things but not at the level you seem to be thinking of, as far as we know.) BTW, I don't know if you're getting confused by negative mass, that is a different hypothetical concept. Nil Einne (talk) 03:10, 13 February 2017 (UTC)
February 13
Lack of certain vitamines and\or minerals can make someone more appetative for eating ?
I know that people who lack Zinc tend to have less appetite. On the contrary, Did a vitamin or mineral that lacking it makes humans to be with more appetite ever found in the seemingly-objective scientific research? 77.180.51.125 (talk) 09:11, 13 February 2017 (UTC)
- I wonder if there's anything in Pica that will help with this? --TammyMoet (talk) 14:45, 13 February 2017 (UTC)