| Revision as of 21:10, 3 October 2006 edit198.236.64.25 (talk) →See also← Previous edit | Revision as of 21:11, 3 October 2006 edit undo198.236.64.25 (talk) →ReferencesNext edit → | ||
| Line 157: | Line 157: | ||
| ] | ] | ||
| ] | ] | ||
| ]ya... i had my way with Taylors mother. RIGHT on the Land rover hood | |||
| ] | |||
| ] | ] | ||
| ] | ] | ||
Revision as of 21:11, 3 October 2006
This article is about the chemical compound. For the material, see Olefin fiber.In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond. The simplest alkenes, with only one double bond and no other functional groups, form a homologous series of hydrocarbons with the general formula CnH2n.
The simplest alkene is ethylene (C2H4), which has the International Union of Pure and Applied Chemistry (IUPAC) name ethene. Alkenes are also called olefins (an archaic synonym, widely used in the petrochemical industry) or vinyl compounds.
Structure of Alkenes
Shape of alkenes
As predicted by the VSEPR model of electron pair repulsion, the molecular geometry of alkenes includes bond angles about each carbon in a double bond of about 120°. The angle may vary because of steric strain introduced by nonbonded interactions created by functional groups attached to the carbons of the double bond. For example, the C-C-C bond angle in propylene is 123.9°. The alkene double bond is stronger than a single covalent bond and also shorter with an average bond length of 133 picometres.
Molecular geometry
Like single covalent bonds, double bonds can be described in terms of overlapping atomic orbitals, except that unlike a single bond (which consists of a single sigma bond), a carbon-carbon double bond consists of one sigma bond and one pi bond.
Each carbon of the double bond uses its three sp hybrid orbitals to form sigma bonds to three atoms. The unhybridized 2p atomic orbitals, which lie perpendicular to the plane created by the axes of the three sp hybrid orbitals, combine to form the pi bond.
Because it requires a large amount of energy to break a pi bond (264 kJ/mol in ethylene), rotation about the carbon-carbon double bond is very difficult and therefore severely restricted. As a consequence substituted alkenes may exist as one of two isomers called a cis isomer and a trans isomer. For example, in cis-2-butylene the two methyl substituents face the same side of the double bond and in trans-2-butylene they face the opposite side.
It is certainly not impossible to twist a double bond. In fact, a 90° twist requires an energy approximately equal to half the strength of a pi bond. The misalignment of the p orbitals is less than expected because pyridalization takes place. trans-Cyclooctene is a stable strained alkene and the orbital misalignment is only 19° with a dihedral angle of 137° (normal 120°) and a degree of pyramidalization of 18°. This explains the dipole moment of 0.8 D for this compound (cis-isomer 0.4 D) where a value of zero is expected. The trans isomer of cycloheptene is only stable at low temperatures.
Physical properties
The physical properties of alkenes are comparable with alkanes. The physical state depends on molecular mass. The simplest alkenes, ethylene, propylene and butylene are gases. Linear alkenes of approximately five to sixteen carbons are liquids, and higher alkenes are waxy solids.
Chemical properties
Alkenes are relatively stable compounds, but are more reactive than alkanes. This is compatible with the idea that the carbon-carbon double bond in alkenes is stronger than the carbon-carbon single bond in alkanes, however, as the majority of the reactions of alkenes involve the rupture of this bond to form two new single bonds.
Synthesis
- The most common industrial synthesis path for alkenes is cracking of petroleum.
- Alkenes can be synthesized from alcohols via dehydration that eliminates water. For example, the dehydration of ethanol produces ethylene:
- CH3CH2OH + H2SO4 → CH3CH2OSO3H + H2O → H2C=CH2 + H2SO4 + H2O
- Other alcohol eliminations are the Chugaev elimination and the Grieco elimination in which the alcohol group is converted to a short-lived intermediate first.
- An elimination reaction from an alkyl amine occurs in the Hofmann elimination and the Cope reaction to produce alkenes.
- Catalytic synthesis of higher α-alkenes can be achieved by a reaction of ethylene with the organometallic compound triethylaluminium in the presence of nickel, cobalt or platinum.
- Alkenes scramble in an olefin metathesis.
- Alkenes can be generated from carbonyl compounds, such as an aldehyde or ketone, by a variety of reactions.
- Reaction with alkyl halides in the Wittig reaction
- Reaction with a phenyl sulfone in the Julia olefination
- Reaction of two different ketones in the Barton-Kellogg reaction
- Coupling of one ketone in the Bamford-Stevens reaction or the Shapiro reaction
- Coupling of two ketones or two aldehydes in the McMurry reaction
- Alkenes can be generated from coupling reactions of vinyl halides.
- Alkenes can be generated by the selective reduction of alkynes.
- Alkenes rearrange in the Diels-Alder reaction and an Ene reaction.
- Alkenes are generated from α-halo sulfones in the Ramberg-Bäcklund Reaction.
Reactions
Alkenes serve as a feedstock for the petrochemical industry because they can participate in a wide variety of reactions.
Addition reactions
Alkenes react in many addition reactions.
- Catalytic addition of hydrogen: Catalytic hydrogenation of alkenes produces the corresponding alkanes. The reaction is carried out under pressure in the presence of a metallic catalyst. Common industrial catalysts are based on platinum, nickel or palladium. For laboratory syntheses, Raney nickel is often employed. This is an alloy of nickel and aluminium. An example of this reaction is the catalytic hydrogenation of ethylene to yield ethane:
- CH2=CH2 + H2 → CH3-CH3
- Electrophilic addition: Most addition reactions to alkenes follow the mechanism of electrophilic addition. An example is the Prins reaction where the electrophile is a carbonyl group.
- Halogenation: Addition of elementary bromine or chlorine to alkenes yields vicinal dibromo- and dichloroalkanes, respectively. The decoloration of a solution of bromine in water is an analytical test for the presence of alkenes:
- CH2=CH2 + Br2 → BrCH2-CH2Br
- It is also used as a quantitive test of unsaturation, expressed as the bromine number of a single compound or mixture.
- This is the mechanism for the reaction:
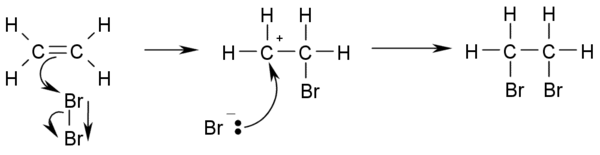
- The reaction works because the high electron density at the double bond causes a temporary shift of electrons in the Br-Br bond causing a temporary induced dipole. This makes the Br closest to the double bond slightly positive and therefore an electrophile.
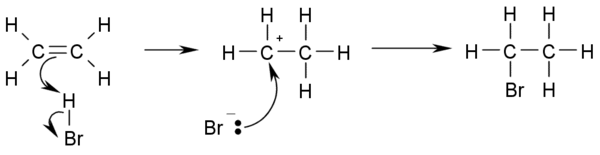
- Hydrohalogenation: Addition of hydrohalic acids such as HCl or HBr to alkenes yields the corresponding haloalkanes.
- CH3-CH=CH2 + HBr → CH3-CHBr-CH3
- If the two carbon atoms at the double bond are linked to a different number of hydrogen atoms, the halogen is found preferentially at the carbon with less hydrogen substituents (Markovnikov's rule).
- This is the reaction mechanism for hydrohalogenation:
- Addition of a carbene or carbenoid yields the corresponding cyclopropane.
Oxidation
Alkenes are oxidized with a large number of oxidizing agents.
- In the presence of oxygen, alkenes burn with a bright flame to produce carbon dioxide and water.
- Catalytic oxidation with oxygen or the reaction with percarboxylic acids yields epoxides
- Reaction with ozone in ozonolysis leads to the breaking of the double bond, yielding two aldehydes or ketones
- R1-CH=CH-R2 + O3 → R1-CHO + R2-CHO + H2O
- This reaction can be used to determine the position of a double bond in an unknown alkene.
- Sharpless bishydroxylation and the Woodward cis-hydroxylation give diols
Polymerization
Polymerization of alkenes is an economically important reaction which yields polymers of high industrial value, such as the plastics polyethylene and polypropylene. Polymerization can either proceed via a free-radical or an ionic mechanism.
Nomenclature of Alkenes
IUPAC Names
To form the root of the IUPAC names for alkenes, simply change the -an- infix of the parent to -en-. For example, CH3-CH3 is the alkane ethANe. The name of CH2=CH2 is therefore ethENe.
In higher alkenes, where isomers exist that differ in location of the double bond, the following numbering system is used:
- Number the longest carbon chain that contains the double bond in the direction that gives the carbon atoms of the double bond the lowest possible numbers.
- Indicate the location of the double bond by the location of its first carbon
- Name branched or substituted alkenes in a manner similar to alkanes.
- Number the carbon atoms, locate and name substituent groups, locate the double bond, and name the main chain
|
CH3CH2CH2CH2CH==CH2 |
CH3 |
CH3 |
Common Names
Despite the precision and universal acceptance of the IUPAC naming system, some alkenes are known almost exclusively by their common names:
| CH2="CH2" | CH3CH="CH2" | CH3C(CH3)="CH2" | |||||||||||||||||||||||||||||||||
| IUPAC name: | Ethene | Propene | 2-Methylpropene | ||||||||||||||||||||||||||||||||
| Common name: | Ethylene | Propylene | Isobutylene
See also
taylor IS A FAGGOT References
what an ass |
||||||||||||||||||||||||||||||||