| Revision as of 11:27, 14 March 2020 editCitation bot (talk | contribs)Bots5,445,340 editsm Alter: journal. | You can use this bot yourself. Report bugs here. | Activated by User:AManWithNoPlan | All pages linked from User:AManWithNoPlan/sandbox2 | via #UCB_webform_linked← Previous edit | Revision as of 16:58, 5 June 2020 edit undoFswitzer4 (talk | contribs)Extended confirmed users10,611 editsm Validated CAS and added FDA UNIINext edit → | ||
| Line 8: | Line 8: | ||
| | Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| | CASNo = 27519-02-4 | | CASNo = 27519-02-4 | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | UNII = 6BSP6HFW73 | |||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | PubChem = 5365075 | | PubChem = 5365075 | ||
| | ChemSpiderID = 4517167 | | ChemSpiderID = 4517167 | ||
Revision as of 16:58, 5 June 2020
| Names | |
|---|---|
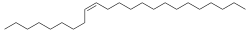
| Preferred IUPAC name (9Z)-Tricos-9-ene | |
| Other names
(Z)-Tricos-9-ene Muscalure | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.044.081 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C23H46 |
| Molar mass | 322.621 g·mol |
| Density | 0.806 g/mL |
| Boiling point | 300 °C (572 °F; 573 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
(Z)-9-Tricosene, known as muscalure, is an insect pheromone found in dipteran flies such as the housefly. Females produce it to attract males to mate. It is used as a pesticide, as in Maxforce Quickbayt by Bayer, luring males to traps to prevent them from reproducing.
Biological functions
(Z)-9-Tricosene is a sex pheromone produced by female house flies (Musca domestica) to attract males. In bees, it is one of the communication pheromones released during the waggle dance.
Uses
As a pesticide, (Z)-9-tricosene is used in fly paper and other traps to lure male flies, trap them, and prevent them from reproducing.
Biosynthesis
(Z)-9-Tricosene is biosynthesized in house flies from nervonic acid. The acid is converted into the acyl-CoA derivative and then reduced to the aldehyde (Z)-15-tetracosenal. Through a decarboxylation reaction, the aldehyde is converted to (Z)-9-tricosene. The process is mediated by a cytochrome P450 enzyme and requires oxygen (O2) and nicotinamide adenine dinucleotide phosphate (NADPH).

Biosynthesis of (Z)-9-tricosene (bottom) from nervonic acid (top)
Safety
Products containing (Z)-9-tricosene are considered safe for humans, wildlife, and the environment.
References
- ^ "(Z)-9-Tricosene". Sigma-Aldrich.
- Thom, C.; Gilley, D.; Hooper, J.; Esch, H. (2007). "The scent of the waggle dance". PLOS Biology. 5 (9): e228. doi:10.1371/journal.pbio.0050228. PMC 1994260. PMID 17713987.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "(Z)-9-Tricosene (103201) Fact Sheet". United States Environmental Protection Agency.
- Reed, JR; Vanderwel, D; Choi, S; Pomonis, JG; Reitz, RC; Blomquist, GJ (1994). "Unusual mechanism of hydrocarbon formation in the housefly: Cytochrome P450 converts aldehyde to the sex pheromone component (Z)-9-tricosene and CO2" (PDF). Proceedings of the National Academy of Sciences of the United States of America. 91 (21): 10000–4. doi:10.1073/pnas.91.21.10000. PMC 44945. PMID 7937826.