This is an old revision of this page, as edited by 70.176.165.122 (talk) at 01:09, 15 December 2011 (→Production of the disulfite ion). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 01:09, 15 December 2011 by 70.176.165.122 (talk) (→Production of the disulfite ion)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Not to be confused with Bisulfite.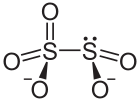
| |
| Names | |
|---|---|
| IUPAC name disulfite | |
| Other names
metabisulfite ion pyrosulfite | |
| Identifiers | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
| Properties | |
| Chemical formula | S2O5 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
A disulfite, commonly known as metabisulfite, is a chemical compound containing the disulfite ion (metabisulfite ion) .
Chemistry
balls tee hee
Other reactions
In aqueous solution, disulfite salts decompose with acids:
S2O5 + H → HSO3 + SO2
Examples of disulfites
- sodium metabisulfite (E223) and potassium metabisulfite (E224) are used as a preservative and antioxidant in food.
References
- International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. p. 130. Electronic version.