This is an old revision of this page, as edited by Beetstra (talk | contribs) at 15:18, 16 February 2012 (Saving copy of the {{chembox}} taken from revid 465128313 of page 2-Butanol for the Chem/Drugbox validation project (updated: '').). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 15:18, 16 February 2012 by Beetstra (talk | contribs) (Saving copy of the {{chembox}} taken from revid 465128313 of page 2-Butanol for the Chem/Drugbox validation project (updated: '').)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| This page contains a copy of the infobox ({{chembox}}) taken from revid 465128313 of page 2-Butanol with values updated to verified values. |
| |
| Names | |
|---|---|
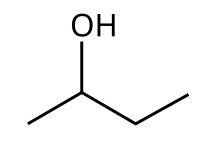
| IUPAC name Butan-2-ol | |
| Other names
sec-Butanol sec-Butyl alcohol | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) | |
| Beilstein Reference | 773649 1718764 (R) |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| Gmelin Reference | 1686 396584 (R) |
| MeSH | 2-butanol |
| PubChem CID | |
| RTECS number |
|
| UN number | 1120 |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C4H10O |
| Molar mass | 74.123 g·mol |
| Density | 0.808 g cm |
| Melting point | −115 °C; −175 °F; 158 K |
| Solubility in water | 290 g dm |
| log P | 0.683 |
| Vapor pressure | 1.67 kPa (at 20 °C) |
| Refractive index (nD) | 1.3978 (at 20 °C) |
| Thermochemistry | |
| Heat capacity (C) | 197.1 J K mol |
| Std molar entropy (S298) |
213.1 J K mol |
| Std enthalpy of formation (ΔfH298) |
−343.3–−342.1 kJ mol |
| Std enthalpy of combustion (ΔcH298) |
−2.6611–−2.6601 MJ mol |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Warning |
| Hazard statements | H226, H319, H335, H336 |
| Precautionary statements | P261, P305+P351+P338 |
| NFPA 704 (fire diamond) |
 |
| Flash point | 22–27 °C |
| Explosive limits | 1.7–9.8% |
| Related compounds | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
- "2-butanol - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification and Related Records. Retrieved 12 October 2011.
- Alger, Donald B. (1991). "The water solubility of 2-butanol: A widespread error". Journal of Chemical Education. 68 (11). USA: ACS Publications: 939. doi:10.1021/ed068p939.1. Retrieved 12 October 2011.
{{cite journal}}: Unknown parameter|month=ignored (help)