This is an old revision of this page, as edited by Beetstra (talk | contribs) at 19:50, 16 February 2012 (Saving copy of the {{chembox}} taken from revid 477101262 of page Acetic_acid for the Chem/Drugbox validation project (updated: 'KEGG').). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 19:50, 16 February 2012 by Beetstra (talk | contribs) (Saving copy of the {{chembox}} taken from revid 477101262 of page Acetic_acid for the Chem/Drugbox validation project (updated: 'KEGG').)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| This page contains a copy of the infobox ({{chembox}}) taken from revid 477101262 of page Acetic_acid with values updated to verified values. |
| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name Acetic acid | |||
| Systematic IUPAC name Ethanoic acid | |||
| Other names Methanecarboxylic acid | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| Abbreviations | AcOH | ||
| Beilstein Reference | 506007 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| EC Number |
| ||
| Gmelin Reference | 1380 | ||
| IUPHAR/BPS | |||
| MeSH | Acetic+acid | ||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 2789 | ||
InChI
| |||
SMILES
| |||
| Properties | |||
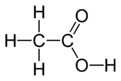
| Chemical formula | C2H4O2 | ||
| Molar mass | 60.052 g·mol | ||
| Appearance | Colourless liquid | ||
| Density | 1.049 g cm | ||
| Solubility in water | Miscible | ||
| log P | -0.322 | ||
| Acidity (pKa) | 4.792 | ||
| Basicity (pKb) | 9.198 | ||
| Viscosity | 1.22 mPa s | ||
| Dipole moment | 1.74 D | ||
| Thermochemistry | |||
| Heat capacity (C) | 123.1 J K mol | ||
| Std molar entropy (S298) |
158.0 J K mol | ||
| Std enthalpy of formation (ΔfH298) |
-483.88--483.16 kJ mol | ||
| Std enthalpy of combustion (ΔcH298) |
-875.50--874.82 kJ mol | ||
| Hazards | |||
| GHS labelling: | |||
| Pictograms |  
| ||
| Signal word | Danger | ||
| Hazard statements | H226, H314 | ||
| Precautionary statements | P280, P305+P351+P338, P310 | ||
| NFPA 704 (fire diamond) |
 | ||
| Flash point | 40 °C | ||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) | 3.31 g kg, oral (rat) | ||
| Related compounds | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Chemical compound
- Scientific literature reviews on generally recognized as safe (GRAS) food ingredients. National Technical Information Service. 1974. p. 1.
- "Chemistry", volume 5, Encyclopedia Britannica, 1961, page 374
- IUPAC, Commission on Nomenclature of Organic Chemistry (1993). "Table 28(a) Carboxylic acids and related groups.Unsubstituted parent structures". A Guide to IUPAC Nomenclature of Organic Compounds (Recommendations 1993). Blackwell Scientific publications.
- "Acetic Acid - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- Cite error: The named reference
BB-prs310305was invoked but never defined (see the help page).