This is an old revision of this page, as edited by Beetstra (talk | contribs) at 08:43, 10 April 2012 (Saving copy of the {{chembox}} taken from revid 482553726 of page Manganese(II)_acetate for the Chem/Drugbox validation project (updated: 'ChemSpiderID', 'StdInChI', 'StdInChIKey').). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 08:43, 10 April 2012 by Beetstra (talk | contribs) (Saving copy of the {{chembox}} taken from revid 482553726 of page Manganese(II)_acetate for the Chem/Drugbox validation project (updated: 'ChemSpiderID', 'StdInChI', 'StdInChIKey').)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| This page contains a copy of the infobox ({{chembox}}) taken from revid 482553726 of page Manganese(II)_acetate with values updated to verified values. |
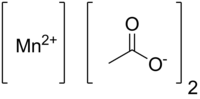
| |
| Names | |
|---|---|
| IUPAC name Manganese(II) acetate | |
| Other names Manganese diacetate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | Mn(CH3COO)2 (anhydrous) Mn(CH3COO)2·4H2O (tetrahydrate) |
| Molar mass | 173.027 g/mol (anhydrous) 245.087 g/mol (tetrahydrate) |
| Appearance | red crystals (anhydrous) red monoclinic crystals (tetrahydrate) |
| Density | 1.74 g/cm (anhydrous) 1.59 g/cm (tetrahydrate) |
| Melting point | 210°C (anhydrous) 80°C (tetrahydrate) |
| Solubility | soluble in water, methanol, acetic acid (anhydrous) soluble in water, ethanol (tetrahydrate) |
| Related compounds | |
| Other anions | Manganese(II) fluoride Manganese(II) chloride Manganese(II) bromide |
| Other cations | Zinc acetate Mercury(II) acetate Silver acetate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Chemical compound
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 3–354, 4–68, ISBN 0849305942