This is an old revision of this page, as edited by Beetstra (talk | contribs) at 11:48, 5 December 2011 (Saving copy of the {{chembox}} taken from revid 433294208 of page Perchloryl_fluoride for the Chem/Drugbox validation project (updated: 'CASNo').). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 11:48, 5 December 2011 by Beetstra (talk | contribs) (Saving copy of the {{chembox}} taken from revid 433294208 of page Perchloryl_fluoride for the Chem/Drugbox validation project (updated: 'CASNo').)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| This page contains a copy of the infobox ({{chembox}}) taken from revid 433294208 of page Perchloryl_fluoride with values updated to verified values. |
| |||
| Names | |||
|---|---|---|---|
| IUPAC name Perchloryl fluoride | |||
| Other names Chlorine oxyfluoride, Perchlorofluoride, Chlorine fluorine oxide, Trioxychlorofluoride, Perchloric acid fluoride | |||
| Identifiers | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| PubChem CID | |||
| RTECS number |
| ||
InChI
| |||
SMILES
| |||
| Properties | |||
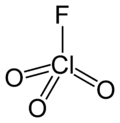
| Chemical formula | ClFO3 | ||
| Molar mass | 102.4496 g/mol | ||
| Appearance | Colorless gas | ||
| Density | 1.4 g/cm | ||
| Melting point | −147.8 °C (−234.0 °F; 125.3 K) | ||
| Boiling point | −46.7 °C (−52.1 °F; 226.5 K) | ||
| Solubility in water | 0.06 g/100 ml (20 °C) | ||
| Structure | |||
| Molecular shape | Tetrahedral | ||
| Thermochemistry | |||
| Std enthalpy of formation (ΔfH298) |
−5.7 | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | Corrosive, oxidizing, toxic | ||
| NFPA 704 (fire diamond) |
 | ||
| Threshold limit value (TLV) | 3 ppm | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Chemical compound
- ^ Harry Julius Emeléus; A. G. Sharpe (1976). Advances in inorganic chemistry and radiochemistry, Volume 18. Academic Press. ISBN 0120236184.