This is an old revision of this page, as edited by Beetstra (talk | contribs) at 11:03, 6 December 2011 (Saving copy of the {{chembox}} taken from revid 464327279 of page Methane for the Chem/Drugbox validation project (updated: 'KEGG').). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 11:03, 6 December 2011 by Beetstra (talk | contribs) (Saving copy of the {{chembox}} taken from revid 464327279 of page Methane for the Chem/Drugbox validation project (updated: 'KEGG').)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| This page contains a copy of the infobox ({{chembox}}) taken from revid 464327279 of page Methane with values updated to verified values. |
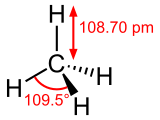
| |||
| |||
| Names | |||
|---|---|---|---|
IUPAC name
| |||
Other names
| |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| Beilstein Reference | 1718732 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
| Gmelin Reference | 59 | ||
| MeSH | Methane | ||
| PubChem CID | |||
| RTECS number |
| ||
| UN number | 1971 | ||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | CH4 | ||
| Molar mass | 16.043 g·mol | ||
| Appearance | Colorless gas | ||
| Odor | Odorless | ||
| Density | 655.6 μg cm | ||
| Melting point | −187.2 °C; −304.9 °F; 86.0 K | ||
| Boiling point | −162 °C; −260 °F; 111 K | ||
| Solubility in water | 35 mg dm (at 17 °C) | ||
| log P | 1.09 | ||
| Thermochemistry | |||
| Heat capacity (C) | 35.69 J K mol | ||
| Std molar entropy (S298) |
186.25 J K mol | ||
| Std enthalpy of formation (ΔfH298) |
−74.87 kJ mol | ||
| Std enthalpy of combustion (ΔcH298) |
−891.1–−890.3 kJ mol | ||
| Hazards | |||
| GHS labelling: | |||
| Pictograms | 
| ||
| Signal word | Danger | ||
| Hazard statements | H220, H280 | ||
| Precautionary statements | P210, P410+P403 | ||
| NFPA 704 (fire diamond) |
 | ||
| Flash point | −188 °C | ||
| Explosive limits | 5–15% | ||
| Related compounds | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Chemical compound
- ^ Linstrom, P.J.; Mallard, W.G., eds. (2011). "Methane". NIST Chemistry WebBook, NIST Standard Reference Database Number 69. National Institute of Standards and Technology. Retrieved 4 December 2011.
- ^ "methane (CHEBI:16183)". Chemical Entities of Biological Interest. UK: European Bioinformatics Institute. 17 October 2009. Main. Retrieved 10 October 2011.
- Matheson Tri-Gas (Dec 4, 2009). "Safety Data Sheet: Methane" (PDF). Matheson Tri-Gas. Retrieved 4 December 2011.

