< Misplaced Pages:WikiProject Chemicals Chembox validation This is an old revision of this page, as edited by Beetstra talk | contribs ) at 12:33, 15 February 2012 (Saving copy of the {{chembox}} taken from revid 473697840 of page Sulfamic_acid for the Chem/Drugbox validation project (updated: '').). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision .
Revision as of 12:33, 15 February 2012 by Beetstra talk | contribs ) (Saving copy of the {{chembox}} taken from revid 473697840 of page Sulfamic_acid for the Chem/Drugbox validation project (updated: '').)(diff ) ← Previous revision | Latest revision (diff ) | Newer revision → (diff )
Sulfamic acid
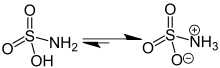
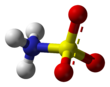
Ball-and-stick model of the canonical neutral form Ball-and-stick model of the zwitterionic form
Names
IUPAC name
Sulfamic acid
Identifiers
CAS Number
3D model (JSmol )
ChEBI
ChEMBL
ChemSpider
EC Number
PubChem CID
RTECS number
UN number
2967
InChI
InChI=1S/H3NO3S/c1-5(2,3)4/h(H3,1,2,3,4)Key: IIACRCGMVDHOTQ-UHFFFAOYSA-N InChI=1/H3NO3S/c1-5(2,3)4/h(H3,1,2,3,4)Key: IIACRCGMVDHOTQ-UHFFFAOYAK
SMILES
Properties
Chemical formula
H3 NSO3
Molar mass
97.10 g/mol
Density
2.15 g/cm
Melting point
205 °C decomp.
Solubility in water
moderate, with slow hydrolysis
Acidity (pK a )
1.0
Related compounds
Other cations
Ammonium sulfamate
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
verify (what is ?)
Infobox references
Tracking categories (test):
cite doi }}) is deprecated. To cite the publication identified by doi:10.1039/JR9600004236, please use {{cite journal }} (if it was published in a bona fide academic journal, otherwise {{cite report }} with |doi=10.1039/JR9600004236 instead.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.
**DISCLAIMER** We are not affiliated with Wikipedia, and Cloudflare.
The information presented on this site is for general informational purposes only and does not constitute medical advice.
You should always have a personal consultation with a healthcare professional before making changes to your diet, medication, or exercise routine.
AI helps with the correspondence in our chat.
We participate in an affiliate program. If you buy something through a link, we may earn a commission 💕
↑