This is an old revision of this page, as edited by 89.240.140.255 (talk) at 14:40, 11 July 2015 (synthesis notes). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 14:40, 11 July 2015 by 89.240.140.255 (talk) (synthesis notes)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Pharmaceutical compound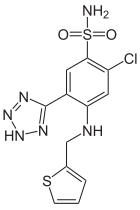 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.044.121 |
| Chemical and physical data | |
| Formula | C12H11ClN6O2S2 |
| Molar mass | 370.84 g/mol g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Azosemide is a high-ceiling diuretic agent.
Synthesis

Chlorosulfonation of 2-fluoro-4-chloro-benzonitrile (1) followed by ammonolysis of the product gives sulfonamide (2). The regiochemistry of the next reaction, nucleophilic aromatic displacement, can be attributed in this case to the better leaving group properties of fluoride ions over chloride ions. Reaction with 2-methylaminothiophene thus gives 3 as the product. There is ample precedent to indicate that tetrazoles are bioisosteric with carboxylic acids, with the two groups showing quite comparable pKAs. Treatment with sodium azide and hydrochloric acid leads to 1,3 addition of the elements of hydrazoic acid to the nitrile and the formation of a tetrazole ring. This yields the high ceiling diuretic agent azosemide (4).
References
- A. Popelak et al., DE 1815922 ; eidem, U.S. patent 3,665,002 (1968, 1972 both to Boehringer, Mann.).
- DE 2353388
This drug article relating to the cardiovascular system is a stub. You can help Misplaced Pages by expanding it. |