This is the current revision of this page, as edited by ItzSwirlz (talk | contribs) at 14:16, 29 March 2024 (Add image). The present address (URL) is a permanent link to this version.
Revision as of 14:16, 29 March 2024 by ItzSwirlz (talk | contribs) (Add image)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)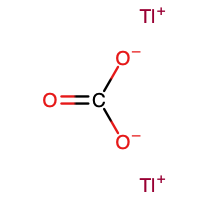 | |
| Names | |
|---|---|
| Other names thallium monocarbonate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.026.759 |
| EC Number |
|
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 1707 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | Tl2CO3 |
| Molar mass | 468.776 g/mol |
| Appearance | white crystals |
| Odor | odorless |
| Density | 7.11 g/cm, solid |
| Melting point | 272 °C (522 °F; 545 K) |
| Solubility in water | 5.2 g/100 mL (25 °C) 27.2 g/100 mL (100 °C) |
| Solubility | insoluble in alcohol, ether, acetone |
| Magnetic susceptibility (χ) | −101.6·10 cm/mol |
| Structure | |
| Crystal structure | monoclinic |
| Hazards | |
| GHS labelling: | |
| Pictograms |   
|
| Signal word | Danger |
| Hazard statements | H300, H330, H373, H411 |
| Precautionary statements | P260, P264, P270, P271, P273, P284, P301+P310, P304+P340, P310, P314, P320, P330, P391, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) |
 |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 21 mg/kg (mouse, oral) |
| LDLo (lowest published) | 23 mg/kg (rat, oral) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Thallium(I) carbonate is the inorganic compound with the formula Tl2CO3. It is a white, water-soluble salt. It has no or very few commercial applications. It is produced by treatment of thallous hydroxide with CO2.
Safety
Like other thallium compounds, it is extremely toxic, with an oral median lethal dose of 21 mg/kg in mice. Due to its toxicity, it is listed in the United States List of Extremely Hazardous Substances as of 2007.
References
- ^ "Thallium (soluble compounds, as Tl)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- "Thallous carbonate". pubchem.ncbi.nlm.nih.gov. Retrieved 12 December 2021.
- Micke, Heinrich; Wolf, Hans Uwe (2000). "Thallium and Thallium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a26_607. ISBN 978-3-527-30673-2.
- "Emergency First Aid Treatment Guide THALLOUS CARBONATE". Chemical Emergency Preparedness and Prevention. U.S. Environmental Protection Agency. Retrieved 2 June 2012.
| Thallium compounds | |||
|---|---|---|---|
| Neg. ox. states | |||
| Thallium(I) |
| ||
| Thallium(III) | |||
| Compounds containing the carbonate group | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |