This is the current revision of this page, as edited by Citation bot (talk | contribs) at 18:37, 24 April 2024 (Removed parameters. | Use this bot. Report bugs. | Suggested by AManWithNoPlan | #UCB_toolbar). The present address (URL) is a permanent link to this version.
Revision as of 18:37, 24 April 2024 by Citation bot (talk | contribs) (Removed parameters. | Use this bot. Report bugs. | Suggested by AManWithNoPlan | #UCB_toolbar)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Chemical compound Pharmaceutical compound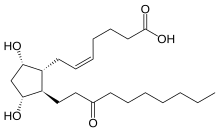 | |
| Clinical data | |
|---|---|
| Trade names | Rescula |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | Topical (eye drops) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 14 min |
| Excretion | Renal |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.227.145 |
| Chemical and physical data | |
| Formula | C22H38O5 |
| Molar mass | 382.541 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Unoprostone (INN) is a prostaglandin analogue. Its isopropyl ester, unoprostone isopropyl, was marketed under the trade name Rescula for the management of open-angle glaucoma and ocular hypertension.
It was approved by the Food and Drug Administration in 2000.
In 2009, Sucampo Pharmaceuticals acquired the rights to the drug in the U.S. and Canada.
In 2015, the drug was discontinued in the U.S.
References
- Micromedex Detailed Consumer Information
- Fung DS, Whitson JT (2014). "An evidence-based review of unoprostone isopropyl ophthalmic solution 0.15% for glaucoma: place in therapy". Clinical Ophthalmology. 8. Auckland, N.Z.: 543–54. doi:10.2147/OPTH.S41562. PMC 3958522. PMID 24648719.
- "Drug Approval Package". Food and Drug Administration.
- "Sucampo Pharmaceuticals, Inc. Acquires Rights to Rescula for U.S. and Canada" (Press release). Business Wire. April 24, 2009.
| Drugs used for glaucoma preparations and miosis (S01E) | |||||||
|---|---|---|---|---|---|---|---|
| Sympathomimetics | |||||||
| Parasympathomimetics |
| ||||||
| Carbonic anhydrase inhibitors/ (sulfonamides) | |||||||
| Beta blocking agents | |||||||
| Prostaglandin analogues (F2α) | |||||||
| Other agents | |||||||
| Eicosanoids | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Precursor | |||||||||||||||
| Prostanoids |
| ||||||||||||||
| Leukotrienes (LT) |
| ||||||||||||||
| Eoxins (EX) |
| ||||||||||||||
| Nonclassic |
| ||||||||||||||
| By function | |||||||||||||||
This pharmacology-related article is a stub. You can help Misplaced Pages by expanding it. |