This is the current revision of this page, as edited by Tom.Reding (talk | contribs) at 10:56, 13 September 2024 (WP:STUBSPACING followup). The present address (URL) is a permanent link to this version.
Revision as of 10:56, 13 September 2024 by Tom.Reding (talk | contribs) (WP:STUBSPACING followup)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) For Indian IT Company Abcence, see Abcence. For the Australian TV station, see ABN (TV station).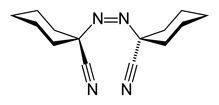
| |
| |

| |
| Names | |
|---|---|
| Systematic IUPAC name 1,1′-Diazene-1,2-diyldicyclohexanecarbonitrile | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Abbreviations | ACHN |
| Beilstein Reference | 960744 |
| ChemSpider | |
| ECHA InfoCard | 100.016.595 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| UN number | 3226 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C14H20N4 |
| Molar mass | 244.342 g·mol |
| Melting point | 114 to 118 °C (237 to 244 °F; 387 to 391 K) decomposes near 80 °C |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Danger |
| Hazard statements | H242, H315, H319, H335 |
| Precautionary statements | P261, P305+P351+P338 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
1,1′-Azobis(cyclohexanecarbonitrile) or ACHN is a radical initiator. The molecular formula is NCC6H10N=NC6H10CN. It is a white solid that is soluble in aromatic solvents.
ACHN has a 10-hour half-life in toluene at 88 °C.
See also
- Azobisisobutylonitrile (AIBN) is another commonly used free radical initiator
References
- ^ 1,1′-Azobis(cyclohexanecarbonitrile) at Sigma-Aldrich
- Steven A. Kates, Fernando Albericio (2001). "1,1'-Azobis-1-cyclohexanenitrile". E-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.ra120.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |