This is an old revision of this page, as edited by Graham Beards (talk | contribs) at 18:58, 22 November 2007 (Tweak). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 18:58, 22 November 2007 by Graham Beards (talk | contribs) (Tweak)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)Template:Taxobox begin
Template:Taxobox image
Template:Taxobox begin placement virus
Template:Taxobox group iii entry
Template:Taxobox familia entry
Template:Taxobox genus entry
Template:Taxobox end placement
Template:Taxobox section subdivision
Rotavirus A (RV-A)
Rotavirus B (RV-B)
Rotavirus C (RV-C)
Rotavirus D (RV-D)
Rotavirus E (RV-E)
Rotavirus F (RV-F)
Rotavirus G (RV-G)
Template:Taxobox end
Rotavirus is the most common cause of viral gastroenteritis in infants and all children suffer from at least one rotavirus infection before their fifth birthday. Rotavirus is found in all countries of the world. In the developing countries approximately 100 million cases of rotavirus infection occur annually in children under five and around 600,000 die. In America, rotaviruses cause an estimated one million episodes of gastroenteritis and 150 deaths of children every year.
Rotavirus is an RNA virus and seven species have been identified. Rotavirus A is the species that causes over 90% of infections in humans. There are different strains of Rotavirus A and many children suffer from more than one infection during their early years. The infection is spread via the fecal-oral route and huge numbers of rotaviruses are excreted by infected children. The virus has a triple-layered protein coat and is very stable outside the body.
All strains of rotavirus infect the cells of small intestine and produce a powerful enterotoxin that causes diarhoea and spreads the infection rapidly within families and communities.
The virus is also important in veterinary medicine, as it infects the young of many animals. As with Influenza, animals infected by rotavirus may act as a reservoir for new strains of rotavirus that could cause epidemics.
In 2003 the World Health Organization and the US Centers for Disease Control established the Rotavirus Vaccine Program to reduce child morbidity and mortality from diarrhoeal disease by making a vaccine against rotavirus available for use in developing countries.
Etymology

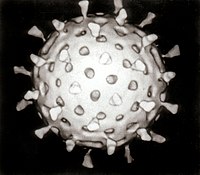
The name rotaviruses was first suggested in 1977 by Thomas Henry Flewett because of the resemblance of rotavirus particles to wheels when they are examined by transmission electron microscopy. The name was later adopted by the International Committee on Taxonomy of Viruses (ICTV) as the official name of the genus. The name is derived from the Latin word for a wheel; rota.
History
In 1943 Light and Hodes described a virus, now known to be a rotavirus, that caused scours in cattle. In the 1960s, a similar virus that infects mice was discovered, which was also subsequently shown to be a rotavirus. In 1973 rotaviruses were first seen in children with gastroenteritis by a research team, lead by Ruth Bishop, in Australia. In the same year, similar discoveries were reported from England by a research group led by Tom Flewett. In 1976 rotaviruses were discovered in several species of animals, and they were soon recognised as a world-wide human and animal pathogen. In the early 1980s rotaviruses were discovered to be a diverse group of viruses. At this time, progress in research was slow because rotaviruses could not be grown artificially in cell cultures. This problem was solved in 1981 by Japanese scientists who successfully grew rotaviruses from humans in monkey kidney cells by adding trypsin to the culture medium. By the mid-1980s the first candidate vaccines were being evaluated.
Microbiology
Types of rotavirus

Rotaviruses are not a single type of virus but are a diverse mixture. There are seven groups which are called rotavirus A, B, C, D, E, F, and G. Groups A, B and C infect humans and all groups can cause disease in animals. Group A rotavirus is by far the commonest cause of infections of humans. Within Group A rotaviruses there is further diversity and different strains, called serotypes, exist. As is the case with Influenza virus, two genes determine rotavirus serotypes and these genes are inherited independently. For group A rotaviruses these genes produce two proteins called VP4 and VP7 which are on the surface of the virion and are antigens. Both of these proteins are important in immunity. Rotavirus A is subdivided into the different serotypes based on the antibody response to VP7 (designated as G types) and to VP4 (P types).
Structure
The genome of rotavirus consists of eleven double helix molecules of RNA which are 18,555 nucleoside base-pairs in total. Each helix, or segment, is a gene and they are numbered 1 to 11 by decreasing size. Each gene codes for one protein except genes 9 and 11 which each code for two. The RNA is surrounded by a three-layered icosahedral protein capsid. The first layer is formed by the protein VP2, with each vertex having a copy of the proteins VP1 and VP3. VP stands for Viral Protein. The second layer is formed by the protein VP6. The outermost protein layer is composed of the structural glycoprotein VP7 and the spike protein VP4. Viral particles are up to 76.5 nm in diameter.
Rotavirus proteins

There are six viral proteins, (VP) which form the virus particle (virion). These are called VP1, VP2, VP3, VP4, VP5, VP6 and VP7 and five non-structural proteins that are only produced in cells infected by rotavirus. These are called NSP1, NSP2, NSP3, NSP4, NSP5 and NSP6. VP1 is located in the core of the virus particle and is an RNA polymerase enzyme. In an infected cell this enzyme produces mRNA transcripts for the synthesis of viral proteins and produces copies of the rotavirus genome RNA segments for newly produced virus particles and VP2 forms the core layer of the virion and binds the RNA genome. VP3 is part of the inner core of the virion but it is also an enzyme called Guanylyl transferase. This is a capping enzyme that catalyses the formation of the 5' cap in the post-transcriptional modification of mRNA. The cap stabilises viral mRNA by protecting it from nucleic acid degrading enzymes called nucleases, and is required for mRNA export to the cytoplasm.
VP4 is a protein on the surface of the virion that protrudes as a spike. VP4 has many functions. It binds to molecules on the surface of cells called receptors and drives the entry of the virus into the cell. VP4 has to be modified by a protease enzyme, (found in the gut), into VP5* and VP8* before the virus is infectious. It also determines how virulent the virus is and along with VP7 determines the serotype of the virus and is important to immunity. VP7 is a glycoprotein that forms the outer surface of the virion. VP6 is the major protein and that forms the bulk of the capsid. It is highly antigenic and can be used to identify rotavirus species as it is conserved within each group. This protein is used in laboratory tests for rotavirus A infections. NSP1 is the product of gene 5, is a nonstructural RNA-binding protein. NSP2 is an RNA-binding protein that accumulates in cytoplasmic inclusions (viroplasms) and is required for genome replication. NSP3 is bound to the end of viral mRNAs in infected cells. NSP4 is a viral enterotoxin to induce diarrhoea and was the first viral enterotoxin discovered. NSP5 is encoded by genome segment 11 of group A rotaviruses and in virus-infected cells NSP5 accumulates in the viroplasms. Gene 11 also encodes NSP6, from an out of phase open reading frame. NSP6 is a nucleic acid binding protein.
| RNA Segment (Gene) | Size (base pairs) | Protein | Molecular Weight kDa | Location | Function |
|---|---|---|---|---|---|
| 1 | 3302 | VP1 | 125 | At the vertices of the core | RNA-dependent RNA polymerase |
| 2 | 2690 | VP2 | 102 | Forms inner shell of the core | Stimulates viral RNA replicase |
| 3 | 2591 | VP3 | 88 | At the vertices of the core | Guanylyl transferase mRNA capping enzyme |
| 4 | 2362 | VP4 | 87 | Surface spike | Cell attachment, virulence, |
| 5 | 1611 | NSP1 | 59 | Non-structural | Not essential to virus growth |
| 6 | 1356 | VP6 | 45 | Inner Capsid | Structural and group antigen |
| 7 | 1104 | NSP3 | 37 | Non-structural | Enhances viral mRNA translation |
| 8 | 1059 | NSP2 | 35 | Non-structural | NTPase involved in RNA packaging |
| 9 | 1062 | VP7 VP7 | 38 and 34 | Surface | Structural and neutralisation antigen |
| 10 | 751 | NSP4 | 20 | Non-structural | Enterotoxin |
| 11 | 667 | NSP5 NSP6 | 22 | Non-structural | ssRNA and dsRNA binding modulator of NSP2 |
This table is based on the simian rotavirus strain SA11. RNA-protein coding assignments differ in some strains.
Replication

Rotaviruses infect the cells that line the small intestine. Their triple protein coats make them very resistant to the acidic pH of the stomach, and also the digestive enzymes (lipases and proteases) in the gut.
They enter cells by endocytosis and form a vesicle known as an endosome. Proteins in the third layer (VP7 and the VP4 spike) disrupt the membrane of the endosome, creating a difference in the Ca concentration. This causes the breakdown of VP7 trimers into single protein subunits, leaving the VP2 and VP6 coats around the viral dsRNA, forming a double-layer particle (DLP).
While the eleven dsRNA strands are still within the protection of the two protein shells, the viral RNA-dependent RNA polymerase creates mRNA transcripts of the double-stranded viral genome. By remaining in the core the viral RNA evades innate host immune responses called RNA interference that are triggered by the presence of double-stranded RNA.
During the infection, rotavirus produces mRNA for both protein translation and genome replication. Most of the rotavirus proteins accumulate in structures known as viroplasms, where the RNA is replicated and the DLPs are assembled. Viroplasms are electron-dense structures found as early as two hours after virus infection around the cell nucleus. Viroplasms are viral factories and are thought to be formed by two viral non-structural proteins, NSP5 and NSP2. Inhibition of NSP5 by RNA interference results in a sharp decrease in rotavirus replication. The DLPs migrate to the endoplasmic reticulum where they obtain their third, outer layer (formed by VP7 and VP4). The progeny viruses are released from the cell by lysis.
Pathogenesis
Rotavirus gastroenteritis is a self-limiting, mild to severe disease characterized by vomiting, watery diarrhoea, and low-grade fever. The infective dose is 10–100 infectious viral particles. Large numbers of virus are in the faeces (10–10 infectious particles per ml), and infection can be readily acquired through contaminated hands, objects, or utensils.

The virus infects enterocytes of the villi of the small intestine, leading to structural changes of the epithelium and diarrhea. The result of this infection is complex, and it is affected by an interaction of host and viral factors. Rotavirus diarrhoea is caused by several mechanisms which include: malabsorption that occurs secondary to the destruction of enterocytes, a reduced supply of blood to the cells that line the small intestine, an activation of the enteric nervous system, and the flow of fluid into the gut from the tissues and blood that is caused by the rotavirus non-structural protein, NSP4, which is an enterotoxin. NSP4 causes an age and dose-dependent diarrhoea in young rodents that is similar to the natural infection. NSP4 also causes cells to become permeable and damages them. Antibody to NSP4 protects mouse pups from diarrhoea induced by different serotypes of rotaviruses. The incubation period ranges from one to three days. Symptoms often start with vomiting followed by four to eight days of diarrhoea. Recovery is usually complete. However, severe diarrhoea without fluid and electrolyte replacement may result in death.
Epidemiology
"Infantile diarrhoea", "winter diarrhoea", "stomach 'flu", "acute nonbacterial infectious gastroenteritis", and "acute viral gastroenteritis" are other names applied to this disease. Humans of all ages are susceptible to rotavirus infection, but children six months to two years of age, the elderly, and the immunocompromised are particularly susceptible to more severe symptoms. Rotaviruses are transmitted by the fecal-oral route. Person-to-person spread through contaminated hands is probably the most important means by which rotaviruses are transmitted in close communities such as pediatric and geriatric wards, day care centers and family homes. Infected food handlers may contaminate foods that require handling and no further cooking, such as salads, fruits, and hors d'oeuvres. Rotaviruses are quite stable in the environment and have been found in estuary samples at levels as high as 1–5 infectious particles per gallon. Sanitary measures adequate for bacteria and parasites seem to be ineffective in endemic control of rotavirus, as similar incidence of rotavirus infection is observed in countries with both high and low health standards.
Group A rotavirus is endemic worldwide. It is the leading cause of severe diarrhoea among infants and children, being responsible for about 20% of cases, and accounts for about half of the cases requiring hospitalization. Boys are twice as likely to be admitted to hospital than girls, but the reason for this is not understood. Almost every child has been infected with rotavirus by age five. Over three million cases of rotavirus gastroenteritis occur annually in the U.S. In temperate areas, it occurs primarily in the winter, but in the tropics it occurs throughout the year. The number attributable to food contamination is unknown.
Group B rotavirus, also called adult diarrhoea rotavirus or ADRV, has caused major epidemics of severe diarrhoea affecting thousands of persons of all ages in China. In a group B epidemic in China in 1982, more than a million people were affected. Group B rotavirus has also been identified after the Chinese epidemics from Calcutta, India in 1998 and this strain was named CAL. Unlike ADRV, the CAL strain is endemic and does not cause known epidemics.
Group C rotavirus has been associated with rare and sporadic cases of diarrhoea in children in many countries. However, the first outbreaks were reported from Japan and England.
About 100 million rotavirus infections occur every year and two million children are admitted to hospital, approximately 600,000 children die from the infection.
| Rotavirus | Influenza virus |
| Segmented RNA genome | Segmented RNA genome |
| Human and animal hosts | Human and animal hosts |
| VP7 and VP4 serotypes | H and N serotypes |
| Groups A, B, C, D, E, F and G | Groups A, B and C |
| Reassortment common within group | Reassortment common within group |
| Infections are seasonal | Infections are seasonal |
| Rotavirus | Influenza virus |
| Faecal-oral transmission | Respiratory transmission |
| Non-enveloped | Enveloped |
| Children mainly affected | Affects all age groups |
| Gastric symptoms | Respiratory Symptoms |
| Endemic | Endemic, epidemic and pandemic |
Diagnosis

Specific diagnosis of the disease is made by identification of the virus in the patient's stool. Enzyme immunoassay (EIA) is the test most widely used to screen clinical specimens, and several commercial kits are available for group A rotavirus. Electron microscopy and polyacrylamide gel electrophoresis are used in some laboratories in addition or as an alternative to EIA. A reverse transcription-polymerase chain reaction (RT-PCR) has been developed to detect and identify all groups and known serotypes of human rotaviruses.
Treatment
There is no cure for rotavirus, so treatment of the disease is aimed at managing the symptoms. Depending on the severity of these treatment consists of oral rehydration with water, or fluids containing sugars and salts. About one out of every 40 children develops severe enough dehydration to require hospitalisation. In these children fluids are given intravenously or nasogastricly and laboratory tests to monitor the child's electrolyte levels and sugar in the blood are carried out.
Vaccines
In 1998 a rotavirus vaccine was licensed for use in the United States. Clinical trials in the United States, Finland, and Venezuela had found it to be 80 to 100% effective at preventing severe diarrhoea caused by group A rotavirus, and researchers had detected no statistically significant serious adverse effects. The manufacturer of the vaccine, however, withdrew it from the market in 1999, after it was discovered that the vaccine may have contributed to an increased risk for intussusception, or bowel obstruction, in one of every 12,000 vaccinated infants. The experience provoked intense debate about the relative risks and benefits of a rotavirus vaccine. In 2006, two vaccines against Rotavirus infection were shown to be safe and effective in children: Rotarix by GlaxoSmithKline and RotaTeq by Merck. Both are taken orally and contain disabled live virus.

RotaTeq is a live, oral pentavalent vaccine that contains five rotaviruses produced by reassortment. The rotavirus parent strains of the reassortants were isolated from human and bovine hosts. Four reassortant rotaviruses express one of the outer capsid, VP7, proteins (serotypes G1, G2, G3, or G4) from the human rotavirus parent strain and the attachment protein VP4 (type P7) from the bovine rotavirus parent strain. The fifth reassortant virus expresses the attachment protein VP4, (type P1A), from the human rotavirus parent strain and the outer capsid protein VP7 (serotype G6) from the bovine rotavirus parent strain.
In February 2006, the U.S. Food and Drug Administration approved RotaTeq for use in the United States. Merck announced a price of $187.50 for the standard three-dose regimen; this is much more expensive than other standard childhood immunisations and, even allowing for discounts, will probably prevent widespread use of the vaccine in poor countries. However, Merck is selling vaccines at dramatically lower prices in developing world countries and is working with a range of partners including the Rotavirus Vaccine Project, PATH, (Program for Appropriate Technology in Health) and other governmental and non-governmental organisations to develop and implement mechanisms for providing access to this vaccine in the developing world.
Epidemics

Outbreaks of group A rotavirus diarrhea are common among hospitalised infants, young children attending day care centres, and elderly persons in nursing homes. Among adults, multiple foods served in banquets were implicated in two outbreaks. An outbreak due to contaminated municipal water occurred in Colorado, 1981.
Several large epidemics of group B rotavirus involving millions of persons as a result of sewage contamination of drinking water supplies have occurred in China since 1982. Although to date outbreaks caused by group B rotavirus have been confined to mainland China, seroepidemiological surveys have indicated lack of immunity to this group of virus in the US. Recent studies led to the identification of group B rotavirus occurring at a sporadic frequency in Calcutta, India and subsequently from other Asian countries.
Rotavirus C had been previously implicated in rare and isolated cases of gastroenteritis. However, it has caused many epidemics among school children, particularly in Japan.
During 2005 the largest recorded epidemic of diarrhoea occurred in Nicaragua. After a detailed investigation this unusual large and severe outbreak was found to be associated with mutations in the rotavirus A genome, possibly helping the virus escape the prevalent immunity in the population which had no protection against this type. A similar large outbreak occurred in Brazil in 1977.
Complications
Rotavirus infections rarely cause other complications in the well managed child. There are reports in the literature of rare complications involving the central nervous system (CNS) where rotavirus was detected in the fluid of the CNS in cases of encephalitis and meningitis, but these complications are uncommon even in the developing countries. Repeated rotavirus infections may increase the risk of celiac disease in genetically susceptible children. A case-control study of infants with a genetic predisposition for celiac disease observed that the risk of developing the disease increased twofold in children who were infected with rotavirus once and almost fourfold for those who were infected with it multiple times.
Rotavirus infections of animals
Rotaviruses infect and cause diarhoea in the young of many species of animals. They have been shown to commonly infect mammals; apes, cattle, pigs, sheep, rats, cats and dogs, mice, rabbits and birds including chickens and turkeys. These rotaviruses are a potential reservoir for genetic exchange with human rotaviruses. There is evidence that animal rotaviruses can infect humans, either by direct transmission of the virus or by contributing one or several RNA segments to reassortants with human strains. Rotaviruses are a major cause of economic loss to farmers because of costs of treatment associated with high morbidity and mortality rates.
References
- Thouless ME, Bryden AS, Flewett TH; et al. (1977). "Serological relationships between rotaviruses from different species as studied by complement fixation and neutralization". Arch. Virol. 53 (4): 287–94. PMID 68765.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Flewett TH, Woode GN (1978). "The rotaviruses". Arch. Virol. 57 (1): 1–23. PMID 77663.
- Light, J.S. and Hodes, H.L. Studies on epidemic diarrhoea in the newborn: isolation of a filterable agent causing diarrhoea in calves. Am. J. Publ. Health. 33, 1451-4, 1943
- Rubenstein D, Milne RG, Buckland R, Tyrrell DA (1971). "The growth of the virus of epidemic diarrhoea of infant mice (EDIM) in organ cultures of intestinal epithelium". British journal of experimental pathology. 52 (4): 442–5. PMID 4998842.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Woode GN, Bridger JC, Jones JM, Flewett TH, Davies HA, Davis HA, White GB (1976). "Morphological and antigenic relationships between viruses (rotaviruses) from acute gastroenteritis of children, calves, piglets, mice, and foals". Infect. Immun. 14 (3): 804–10. PMID 965097.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Bishop RF, Davidson GP, Holmes IH, Ruck BJ (1973). "Letter: Evidence for viral gastroenteritis". N. Engl. J. Med. 289 (20): 1096–7. PMID 4742237.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Bishop RF, Davidson GP, Holmes IH, Ruck BJ (1973). "Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis". Lancet. 2 (7841): 1281–3. PMID 4127639.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Flewett TH, Bryden AS, Davies H (1973). "Letter: Virus particles in gastroenteritis". Lancet. 2 (7844): 1497. PMID 4129337.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Beards GM, Pilfold JN, Thouless ME, Flewett TH (1980). "Rotavirus serotypes by serum neutralisation". J. Med. Virol. 5 (3): 231–7. PMID 6262451.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Beards GM (1982). "Polymorphism of genomic RNAs within rotavirus serotypes and subgroups". Arch. Virol. 74 (1): 65–70. PMID 6297431.
- Urasawa T, Urasawa S, Taniguchi K (1981). "Sequential passages of human rotavirus in MA-104 cells". Microbiol. Immunol. 25 (10): 1025–35. PMID 6273696.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Vesikari T, Isolauri E, Delem A; et al. (1985). "Clinical efficacy of the RIT 4237 live attenuated bovine rotavirus vaccine in infants vaccinated before a rotavirus epidemic". J. Pediatr. 107 (2): 189–94. PMID 3894608.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Beards GM, Brown DW (1988). "The antigenic diversity of rotaviruses: significance to epidemiology and vaccine strategies". Eur. J. Epidemiol. 4 (1): 1–11. PMID 2833405.
- Santos N, Hoshino Y (2005). "Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine". Rev. Med. Virol. 15 (1): 29–56. doi:10.1002/rmv.448. PMID 15484186.
- Desselberger U, Iturriza-Gómara M, Gray JJ (2001). "Rotavirus epidemiology and surveillance". Novartis Found. Symp. 238: 125–47, discussion 147–52. PMID 11444024.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Chan WK, Penaranda ME, Crawford SE, Estes MK (1986). "Two glycoproteins are produced from the rotavirus neutralization gene". Virology. 151 (2): 243–52. PMID 3010552.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pesavento JB, Crawford SE, Estes MK, Prasad BV (2006). "Rotavirus proteins: structure and assembly". Curr. Top. Microbiol. Immunol. 309: 189–219. PMID 16913048.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Prasad BV, Chiu W (1994). "Structure of rotavirus". Curr. Top. Microbiol. Immunol. 185: 9–29. PMID 8050286.
- Vásquez-del Carpió R, Morales JL, Barro M, Ricardo A, Spencer E (2006). "Bioinformatic prediction of polymerase elements in the rotavirus VP1 protein". Biol. Res. 39 (4): 649–59. doi:/S0716-97602006000500008. PMID 17657346.
{{cite journal}}: Check|doi=value (help)CS1 maint: multiple names: authors list (link) - Arnoldi F, Campagna M, Eichwald C, Desselberger U, Burrone OR (2007). "Interaction of rotavirus polymerase VP1 with nonstructural protein NSP5 is stronger than that with NSP2". J. Virol. 81 (5): 2128–37. doi:10.1128/JVI.01494-06. PMID 17182692.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Fresco LD, Buratowski S (1994). "Active site of the mRNA-capping enzyme guanylyltransferase from Saccharomyces cerevisiae: similarity to the nucleotidyl attachment motif of DNA and RNA ligases". Proc. Natl. Acad. Sci. U.S.A. 91 (14): 6624–8. PMID 8022828.
- Gardet A, Breton M, Fontanges P, Trugnan G, Chwetzoff S (2006). "Rotavirus spike protein VP4 binds to and remodels actin bundles of the epithelial brush border into actin bodies". J. Virol. 80 (8): 3947–56. doi:10.1128/JVI.80.8.3947-3956.2006. PMID 16571811.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Arias CF, Isa P, Guerrero CA, Méndez E, Zárate S, López T, Espinosa R, Romero P, López S (2002). "Molecular biology of rotavirus cell entry". Arch. Med. Res. 33 (4): 356–61. PMID 12234525.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Konno T, Suzuki H, Kitaoka S, Sato T, Fukuhara N, Yoshie O, Fukudome K, Numazaki Y (1993). "Proteolytic enhancement of human rotavirus infectivity". Clin. Infect. Dis. 16 Suppl 2: S92–7. PMID 8384014.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Hoshino Y, Jones RW, Kapikian AZ (2002). "Characterization of neutralization specificities of outer capsid spike protein VP4 of selected murine, lapine, and human rotavirus strains". Virology. 299 (1): 64–71. PMID 12167342.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Beards GM, Campbell AD, Cottrell NR, Peiris JS, Rees N, Sanders RC, Shirley JA, Wood HC, Flewett TH (1984). "Enzyme-linked immunosorbent assays based on polyclonal and monoclonal antibodies for rotavirus detection". J. Clin. Microbiol. 19 (2): 248–54. PMID 6321549.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Hua J, Mansell EA, Patton JT (1993). "Comparative analysis of the rotavirus NS53 gene: conservation of basic and cysteine-rich regions in the protein and possible stem-loop structures in the RNA". Virology. 196 (1): 372–8. doi:10.1006/viro.1993.1492. PMID 8395125.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Kattoura MD, Chen X, Patton JT (1994). "The rotavirus RNA-binding protein NS35 (NSP2) forms 10S multimers and interacts with the viral RNA polymerase". Virology. 202 (2): 803–13. doi:10.1006/viro.1994.1402. PMID 8030243.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Taraporewala ZF, Patton JT (2004). "Nonstructural proteins involved in genome packaging and replication of rotaviruses and other members of the Reoviridae". Virus Res. 101 (1): 57–66. doi:10.1016/j.virusres.2003.12.006. PMID 15010217.
- Poncet D, Aponte C, Cohen J (1993). "Rotavirus protein NSP3 (NS34) is bound to the 3' end consensus sequence of viral mRNAs in infected cells". J. Virol. 67 (6): 3159–65. PMID 8388495.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Dong Y, Zeng CQ, Ball JM, Estes MK, Morris AP (1997). "The rotavirus enterotoxin NSP4 mobilizes intracellular calcium in human intestinal cells by stimulating phospholipase C-mediated inositol 1,4,5-trisphosphate production". Proc. Natl. Acad. Sci. U.S.A. 94 (8): 3960–5. PMID 9108087.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Afrikanova I, Miozzo MC, Giambiagi S, Burrone O (1996). "Phosphorylation generates different forms of rotavirus NSP5". J. Gen. Virol. 77 ( Pt 9): 2059–65. PMID 8811003.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Mohan KV, Atreya CD (2001). "Nucleotide sequence analysis of rotavirus gene 11 from two tissue culture-adapted ATCC strains, RRV and Wa". Virus Genes. 23 (3): 321–9. PMID 11778700.
- Rainsford EW, McCrae MA (2007). "Characterization of the NSP6 protein product of rotavirus gene 11". doi:10.1016/j.virusres.2007.06.011. PMID 17658646.
{{cite journal}}: Cite journal requires|journal=(help) - Desselberger U. Rotavirus: basic facts. In Rotaviruses Methods and Protocols. Ed. Gray, J. and Desselberger U. Humana Press,2000,p1-8.ISBN 0-89603-736-3
- Patton JT. Rotavirus RNA replication and gene expression. In Novartis Foundation. Gastroenteritis Viruses, Humana Press, 2001,p 64-81. ISBN 0-471-49663-4
- Jayaram H, Estes MK, Prasad BV (2004). "Emerging themes in rotavirus cell entry, genome organization, transcription and replication". Virus Res. 101 (1): 67–81. doi:10.1016/j.virusres.2003.12.007. PMID 15010218.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Estes MK, Kang G, Zeng CQ, Crawford SE, Ciarlet M (2001). "Pathogenesis of rotavirus gastroenteritis". Novartis Found. Symp. 238: 82–96, discussion 96–100. PMID 11444037.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Leung AK, Kellner JD, Davies HD (2005). "Rotavirus gastroenteritis". Advances in therapy. 22 (5): 476–87. PMID 16418157.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Dennehy PH (2000). "Transmission of rotavirus and other enteric pathogens in the home". Pediatr. Infect. Dis. J. 19 (10 Suppl): S103–5. PMID 11052397.
- Van Man N, Luan le T, Trach DD; et al. (2005). "Epidemiological profile and burden of rotavirus diarrhea in Vietnam: 5 years of sentinel hospital surveillance, 1998-2003". J. Infect. Dis. 192 Suppl 1: S127–32. doi:10.1086/431501. PMID 16088796.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Koopmans M, Brown D (1999). "Seasonality and diversity of Group A rotaviruses in Europe". Acta paediatrica (Oslo, Norway : 1992). Supplement. 88 (426): 14–9. PMID 10088906.
- ^ Hung T, Chen GM, Wang CG, Yao HL, Fang ZY, Chao TX, Chou ZY, Ye W, Chang XJ, Den SS (1984). "Waterborne outbreak of rotavirus diarrhoea in adults in China caused by a novel rotavirus". Lancet. 1 (8387): 1139–42. PMID 6144874.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid6144874" was defined multiple times with different content (see the help page). - Kelkar SD, Zade JK (2004). "Group B rotaviruses similar to strain CAL-1, have been circulating in Western India since 1993". Epidemiol. Infect. 132 (4): 745–9. PMID 15310177.
- Brown DW, Campbell L, Tomkins DS, Hambling MH (1989). "School outbreak of gastroenteritis due to atypical rotavirus". Lancet. 2 (8665): 737–8. PMID 2570978.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Kuzuya M, Fujii R, Hamano M, Nishijima M, Ogura H (2007). "Detection and molecular characterization of human group C rotaviruses in Okayama Prefecture, Japan, between 1986 and 2005". J. Med. Virol. 79 (8): 1219–28. doi:10.1002/jmv.20910. PMID 17596825.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Widdowson MA, Bresee JS, Gentsch JR, Glass RI (2005). "Rotavirus disease and its prevention". Curr. Opin. Gastroenterol. 21 (1): 26–31. PMID 15687881.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Parashar UD, Gibson CJ, Bresse JS, Glass RI (2006). "Rotavirus and severe childhood diarrhea". Emerging Infect. Dis. 12 (2): 304–6. PMID 16494759.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Fischer TK, Viboud C, Parashar U; et al. (2007). "Hospitalizations and deaths from diarrhea and rotavirus among children <5 years of age in the United States, 1993-2003". J. Infect. Dis. 195 (8): 1117–25. doi:10.1086/512863. PMID 17357047.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Beards GM (1988). "Laboratory diagnosis of viral gastroenteritis". Eur. J. Clin. Microbiol. Infect. Dis. 7 (1): 11–3. PMID 3132369.
- Fischer TK, Gentsch JR (2004). "Rotavirus typing methods and algorithms". Rev. Med. Virol. 14 (2): 71–82. doi:10.1002/rmv.411. PMID 15027000.
- Sachdev HP (1996). "Oral rehydration therapy". Journal of the Indian Medical Association. 94 (8): 298–305. PMID 8855579.
- Patel MM, Tate JE, Selvarangan R, Daskalaki I, Jackson MA, Curns AT, Coffin S, Watson B, Hodinka R, Glass RI, Parashar UD (2007). "Routine laboratory testing data for surveillance of rotavirus hospitalizations to evaluate the impact of vaccination". Pediatr. Infect. Dis. J. 26 (10): 914–9. doi:10.1097/INF.0b013e31812e52fd. PMID 17901797.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Bines J (2006). "Intussusception and rotavirus vaccines". Vaccine. 24 (18): 3772–6. doi:10.1016/j.vaccine.2005.07.031. PMID 16099078.
- O'Ryan M (2007). "Rotarix (RIX4414): an oral human rotavirus vaccine". Expert review of vaccines. 6 (1): 11–9. doi:10.1586/14760584.6.1.11. PMID 17280473.
- Matson DO (2006). "The pentavalent rotavirus vaccine, RotaTeq". Seminars in paediatric infectious diseases. 17 (4): 195–9. doi:10.1053/j.spid.2006.08.005. PMID 17055370.
- McCarthy M (2003). "Project seeks to "fast track" rotavirus vaccine". Lancet. 361 (9357): 582. PMID 12598149.
- Hopkins RS, Gaspard GB, Williams FP, Karlin RJ, Cukor G, Blacklow NR (1984). "A community waterborne gastroenteritis outbreak: evidence for rotavirus as the agent". American journal of public health. 74 (3): 263–5. PMID 6320684.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Fang ZY, Ye Q, Ho MS; et al. (1989). "Investigation of an outbreak of adult diarrhea rotavirus in China". J. Infect. Dis. 160 (6): 948–53. PMID 2555422.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Ahmed MU, Kobayashi N, Wakuda M, Sanekata T, Taniguchi K, Kader A, Naik TN, Ishino M, Alam MM, Kojima K, Mise K, Sumi A (2004). "Genetic analysis of group B human rotaviruses detected in Bangladesh in 2000 and 2001". J. Med. Virol. 72 (1): 149–55. doi:10.1002/jmv.10546. PMID 14635024.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Iizuka S, Tabara K, Kawamukai A, Itogawa H, Hoshina K (2006). "An outbreak of group C rotavirus infection in an elementary school in Shimane prefecture, Japan, February 2006". Jpn. J. Infect. Dis. 59 (5): 350–1. PMID 17060710.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Kuzuya M, Fujii R, Hamano M, Ogura H (2003). "". Kansenshogaku Zasshi (in Japanese). 77 (2): 53–9. PMID 12661079.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Hamano M, Kuzuya M, Fujii R, Ogura H, Mori T, Nakayama T, Yuen E, Katayama K, Mitsunobu Y, Inoue K (1999). "Outbreak of acute gastroenteritis caused by human group C rotavirus in a primary school". Jpn. J. Infect. Dis. 52 (4): 170–1. PMID 10592901.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Bucardo F, Karlsson B, Nordgren J; et al. (2007). "Mutated G4P rotavirus associated with a nationwide outbreak of gastroenteritis in Nicaragua in 2005". J. Clin. Microbiol. 45 (3): 990–7. doi:10.1128/JCM.01992-06. PMID 17229854.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Linhares AC, Pinheiro FP, Freitas RB, Gabbay YB, Shirley JA, Beards GM (1981). "An outbreak of rotavirus diarrhea among a non-immune, isolated South American Indian community". Am. J. Epidemiol. 113 (6): 703–10. PMID 6263087.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ramig RF (2007). "Systemic rotavirus infection". Expert review of anti-infective therapy. 5 (4): 591–612. doi:10.1586/14787210.5.4.591. PMID 17678424.
- Goto T, Kimura H, Numazaki K, Akiyama M, Kato M, Noda M, Nozaki Y, Tanaka-Taya K, Taniguchi K, Yamagata T, Nishio O, Oogane T, Momoi MY, Okabe N (2007). "A case of meningoencephalitis associated with G1P rotavirus infection in a Japanese child": 1–4. doi:10.1080/00365540701466249. PMID 17852929.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - Kehle J, Metzger-Boddien C, Tewald F, Wald M, Schüürmann J, Enders G (2003). "First case of confirmed rotavirus meningoencephalitis in Germany". Pediatr. Infect. Dis. J. 22 (5): 468–70. PMID 12797316.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Pager C, Steele D, Gwamanda P, Driessen M (2000). "A neonatal death associated with rotavirus infection--detection of rotavirus dsRNA in the cerebrospinal fluid". S. Afr. Med. J. 90 (4): 364–5. PMID 10957919.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Stene LC, Honeyman MC, Hoffenberg EJ, Haas JE, Sokol RJ, Emery L, Taki I, Norris JM, Erlich HA, Eisenbarth GS, Rewers M (2006). "Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study". Am. J. Gastroenterol. 101 (10): 2333–40. doi:10.1111/j.1572-0241.2006.00741.x. PMID 17032199.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Ashley CR, Caul EO, Clarke SK, Corner BD, Dunn S (1978). "Rotavirus infections of apes". Lancet. 2 (8087): 477. PMID 79844.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Wani SA, Bhat MA, Ishaq SM, Ashrafi MA (2004). "Determination of bovine rotavirus G genotypes in Kashmir, India". Rev. - Off. Int. Epizoot. 23 (3): 931–6. PMID 15861888.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Saif LJ (1999). "Enteric viral infections of pigs and strategies for induction of mucosal immunity". Advances in veterinary medicine. 41: 429–46. PMID 9890034.
- ^ Holland RE (1990). "Some infectious causes of diarrhea in young farm animals". Clin. Microbiol. Rev. 3 (4): 345–75. PMID 2224836.
- Pérez-Cano FJ, Castell M, Castellote C, Franch A (2007). "Characterization of Clinical and Immune Response in a Rotavirus Diarrhea Model in Suckling Lewis Rats". Pediatr Res. doi:10.1203/PDR.0b013e318159a273. PMID 17957154.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Enriquez C, Nwachuku N, Gerba CP (2001). "Direct exposure to animal enteric pathogens". Reviews on environmental health. 16 (2): 117–31. PMID 11512628.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Feng N, Franco MA, Greenberg HB (1997). "Murine model of rotavirus infection". Adv. Exp. Med. Biol. 412: 233–40. PMID 9192019.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Thouless ME, DiGiacomo RF, Deeb BJ, Howard H (1988). "Pathogenicity of rotavirus in rabbits". J. Clin. Microbiol. 26 (5): 943–7. PMID 2838507.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Guy JS (1998). "Virus infections of the gastrointestinal tract of poultry". Poult. Sci. 77 (8): 1166–75. PMID 9706084.
- Müller H, Johne R (2007). "Rotaviruses: diversity and zoonotic potential--a brief review". Berl. Munch. Tierarztl. Wochenschr. 120 (3–4): 108–12. PMID 17416132.
- Cook N, Bridger J, Kendall K, Gomara MI, El-Attar L, Gray J (2004). "The zoonotic potential of rotavirus". J. Infect. 48 (4): 289–302. doi:10.1016/j.jinf.2004.01.018. PMID 15066329.
{{cite journal}}: CS1 maint: multiple names: authors list (link)