This is an old revision of this page, as edited by Wikkidd (talk | contribs) at 04:06, 4 July 2008 (Undid revision 223453007 by JoshuaZ (talk)). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 04:06, 4 July 2008 by Wikkidd (talk | contribs) (Undid revision 223453007 by JoshuaZ (talk))(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)A diamondoid, in the context of building materials for nanotechnology components, most generally refers to structures that resemble diamond in a broad sense: namely, strong, stiff structures containing dense, 3-D networks of covalent bonds, formed chiefly from first and second row atoms with a valence of three or more. Examples of diamondoid structures would include crystalline diamond, sapphire, and other stiff structures similar to diamond but with various atom substitutions which might include N, O, Si, S, and so forth. Sp²-hybridized carbon structures that - in contrast to sp³-hybridized carbon in diamond - arrange in planar sheets ("graphene" sheets) are sometimes also included in the class of diamondoid materials for nanotechnology, e.g., graphite, carbon nanotubes consisting of sheets of carbon atoms rolled into tubes, spherical buckyballs and other graphene structures.
Chemistry
In the context of classical chemistry, "diamondoid" refers to variants of the carbon cage molecule known as adamantane (C10H16), the smallest unit cage structure of the diamond crystal lattice. Diamondoids also known as nanodiamonds or condensed adamantanes may include one or more cages (adamantane, diamantane, triamantane, and higher polymantanes) as well as numerous isomeric and structural variants of adamantanes and polymantanes. These diamondoids occur naturally in petroleum deposits and have been extracted and purified into large pure crystals of polymantane molecules having more than a dozen adamantane cages per molecule . These species are of interest as molecular approximations of the cubic diamond framework, terminated with C-H bonds. Cyclohexamantane may be thought of as a nanometer-sized diamond of approximately 5.6 * 10 grams.

Examples include:
- Adamantane (C10H16)
- Iceane (C12H18)
- BC-8 (C14H20)
- Diamantane (C14H20) also diadamantane, two face-fused cages
- Triamantane (C18H24), also triadamantane. Diamantane has 4 identical faces available for anchoring a new C4H4 unit.
- Isotetramantane (C22H28). Triamantane has 8 faces on to which a new C4H4 unit can be added resulting in 4 isomers. One of these isomers displays a helical twist and is therefore prochiral. The P and M enantiomers have been separated.
- Pentamantane has 9 isomers with chemical formula C26H32 and one more pentamantane exists with chemical formula C25H30
- Cyclohexamantane (C26H30)
- Super-adamantane (C35H36)
- Basic beryllium acetate Be4O(O2CCH3)6
One tetramantane isomer is the largest ever diamondoid prepared by organic synthesis. The first ever isolation of a wide range of diamondoids from petroleum took place in the following steps : a vacuum distillation above 345 °C, the equivalent atmospheric boiling point, then pyrolysis at 400 to 450 °C in order to remove all non-diamondoid compounds and then a series of HPLC separation techniques.
In one study a tetramantane compound is fitted with thiol groups at the bridgehead positions . This allows their anchorage to a gold surface and formation of self-assembled monolayers (diamond-on-gold).
Organic chemistry of diamondoids even extends to pentamantane . The medial position (base) in this molecule is calculated to yield a more favorable carbocation than the apical position (top) and simple bromination of pentamane 1 with bromine exclusively gives the medial bromo derivative 2 which on hydrolysis in water / DMF forms the alcohol 3.
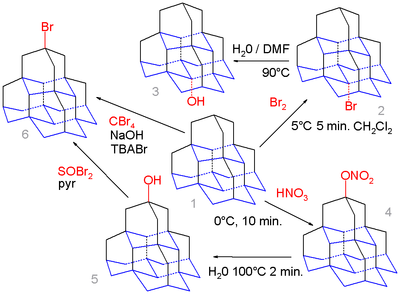
In contrast nitroxylation of 1 with nitric acid gives the apical nitrate 4 as an intermediate which is hydrolyzed to the apical alcohol 5 due to the higher steric demand of the active electrophilic NO2 - HNO3 species. This alcohol can react with thionyl bromide to the bromide 6 and in a series of steps (not shown) to the corresponding thiol. Pentamantane can also react with tetrabromomethane and tetra-n-butylammonium Bromide (TBABr) in a free radical reaction to the bromide but without selectivity.
Origin and occurrence of diamondoids
Diamondoids are found in hydrocarbons (oil and natural gas, mainly in condensates). Condensates, which are extremely light oils, have about a spoonful of diamondoids per gallon (about 3.78 liters). It has been suggested that diamondoids may come with the small amount of hydrocarbons that migrate from the mantle to the Earth's crust. A study by Mello and Moldowan in 2005 clearly shows that diamondoids found in crude oil are derived from a biogenic carbon source.. However, no scientist believes diamondoids or hydrocarbons are formed in the Earth's crust or from biological organisms. No biological molecule can survive 10 kilometers deep at pressures above 30 kilobar.
See also
- Other diamond-like compounds: Boron nitride
External links
- Cluster and Nanocrystal Research Group, Technische Universität Berlin
- Molecular Diamond Technologies, Chevron Texaco
- Nanotechnology and the arrival of the Diamond Age
- Laser Raman Spectroscopy and Modelling of Diamondoids
References
- ^ Isolation and Structure of Higher Diamondoids, Nanometer-Sized Diamond Molecules J. E. Dahl, S. G. Liu, and R. M. K. Carlson Science 3 January 2003 from 299: 96-99; published online 29 November 2002 Abstract
- J. E. P. Dahl, J. M. Moldowan, T. M. Peakman, J. C. Clardy, E. Lobkovsky, M. M. Olmstead, P. W. May, T. J. Davis, J. W. Steeds, K. E. Peters, A. Pepper, A. Ekuan, R. M. K. Carlson (2003). "Isolation and Structural Proof of the Large Diamond Molecule, Cyclohexamantane (C26H30)". Angewandte Chemie International Edition. 42: 2040–2044. doi:10.1002/anie.200250794.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Diamondoids are thermodynamically very stable and will survive this pyrolysis
- Functionalized Nanodiamonds Part 3: Thiolation of Tertiary/Bridgehead Alcohols Boryslav A. Tkachenko, Natalie A. Fokina, Lesya V. Chernish, Jeremy E. P. Dahl, Shenggao Liu, Robert M. K. Carlson, Andrey A. Fokin, and Peter R. Schreiner Org. Lett.; 2006; 8(9) pp 1767 - 1770; (Letter) Graphical abstract
- Reactivity of Pentamantane (Td-Pentamantane): A Nanoscale Model of Diamond Andrey A. Fokin, Peter R. Schreiner, Natalie A. Fokina, Boryslav A. Tkachenko, Heike Hausmann, Michael Serafin, Jeremy E. P. Dahl, Shenggao Liu, and Robert M. K. Carlson J. Org. Chem.; 2006; 71(22) pp 8532 - 8540; (Article) doi:10.1021/jo061561x
- http://www.searchanddiscovery.net/documents/abstracts/2005research_calgary/abstracts/extended/mello/mello.htm
- http://www.pnas.org/content/99/17/10976.full?maxtoshow=&HITS=10&hits=10&RESULTFORMAT=&fulltext=genesis+of+hydrocarbons+and+the+origin+of+petroleum&searchid=1085470440708_510&stored_search=&FIRSTINDEX=0
- http://aapg.confex.com/aapg/2007int/techprogram/A112905.htm
- http://www.gasresources.net/DisposalBioClaims.htm
- http://www.pnas.org/content/99/17/10976.full?maxtoshow=&HITS=10&hits=10&RESULTFORMAT=&fulltext=genesis+of+hydrocarbons+and+the+origin+of+petroleum&searchid=1085470440708_510&stored_search=&FIRSTINDEX=0
- http://aapg.confex.com/aapg/2007int/techprogram/A112905.htm
- http://www.gasresources.net/DisposalBioClaims.htm