This is an old revision of this page, as edited by 220.239.52.128 (talk) at 05:17, 7 September 2008 (→Diagnosis). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 05:17, 7 September 2008 by 220.239.52.128 (talk) (→Diagnosis)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Medical condition| Huntington's disease | |
|---|---|
| Specialty | Neurology |
| Frequency | 0.0123% (United Kingdom) |
Huntington's disease, also called Huntington's chorea, chorea major, or HD, is an inherited genetic neurological disorder characterized by abnormally uncoordinated, jerky body movements called chorea and a decline in some mental abilities, which can lead to affected aspects of behavior. As the disorder progresses, it can cause complications that significantly reduce life expectancy. The exact mechanism of HD is unproven and there is currently no proven cure, so symptoms are managed with a range of medications to treat individual symptoms and supportive services. Global incidence varies, from 3 to 7 per 100,000 people of Western European descent, down to 1 per 1,000,000 of Asian and African descent. The onset of physical symptoms in Huntington's disease occur in a large range around a mean of a person's late forties to early fifties. If symptoms become noticeable before a person is twenty, their condition is known as juvenile HD.
Huntington's disease is one of several polyglutamine diseases caused by the length of a repeated section of a gene exceeding the normal range. The huntingtin gene (HTT) normally provides the information to produce Huntingtin protein, but when affected, produces mutant Huntingtin (mHTT) instead. The presence of mHTT causes an increase in the rate of neuronal cell death in select areas of the brain, affecting neurological functions.
The disorder is named after George Huntington, the American physician who first described it in 1872. In 1983 a marker for the altered DNA causing the disease was found, followed a decade later by discovery of a single, causal, gene. As it was caused by a single gene, an accurate genetic test for HD was developed, this was one of the first inherited genetic disorders for which this was possible. Due to the availability of this test, and similar characteristics with other neurological disorders, HD research has been increasing over time.
Signs and symptoms
Physical symptoms are usually the first to cause problems and to be noticed, but at this point they are usually accompanied by cognitive and psychiatric ones that are often not recognized. Almost everyone with Huntington's disease eventually exhibits all physical symptoms, but cognitive and psychiatric symptoms vary significantly between individuals.
Physical
The most characteristic symptoms are jerky, random, and uncontrollable movements called chorea, although, in a few cases, very slow movement and stiffness called (bradykinesia and dystonia) can occur instead, these can also become more promininent in later stages of the disorder. Abnormal movements are initially exhibited as general lack of coordination, an unsteady gait and slurring of speech, but, as the disease progresses, any function that requires muscle control is affected, causing physical instability, abnormal facial expression, impaired speech comprehensibility and difficulties chewing and swallowing. Eating difficulties commonly cause weight loss and may lead to malnutrition. Associated symptoms involve sleep cycle disturbances, including insomnia and rapid eye movement sleep alterations. Juvenile HD generally progress faster and symptoms more likely to exhibit rigidity and bradykinesia, instead of chorea, and commonly include seizures.
Cognitive
Select cognitive abilities are impaired progressively, these include executive function (planning, cognitive flexibility, abstract thinking, rule acquisition, initiating appropriate actions and inhibiting inappropriate actions), psychomotor function which exhibits as inhibition of thought processes to control muscles, perceptual and spatial skills of both self, and the surrounding environment, selection of correct methods of remembering information (but not actual long-term memory itself), short-term memory, and ability to learn new skills. How much each ability is affected varies depending on each individual's pathology.
Psychiatric
These symptoms vary far more than cognitive and physical ones, and may include anxiety, depression, a reduced display of emotions (blunted affect) and decreased ability to recognize negative expressions like anger, disgust, fear or sadness in others, egocentrism, aggression, and compulsive behavior, which can cause, or worsen addictions, including alcoholism and gambling, or hypersexuality.
Genetics
See also: Trinucleotide repeat disordersThe Huntingtin gene (HTT) is located on the short arm of chromosome 4 (4p16.3). HTT contains a sequence of three DNA bases—cytosine-adenine-guanine (CAG)—repeated multiple times (i.e. ...CAGCAGCAG...) on its 5' end, known as a trinucleotide repeat. CAG is the genetic code for the amino acid glutamine, so a series of them results in the production of a chain of glutamine known as polyglutamine or polyQ.
| Repeat count | Classification | Disease status |
|---|---|---|
| <27 | Normal | Unaffected |
| 27–35 | Intermediate | Unaffected |
| 36–39 | Reduced Penetrance | +/- Affected |
| >39 | Full Penetrance | Affected |
The polyQ region contains fewer than 36 glutamines results in production of the cytoplasmic protein called huntingtin (HTT). In the general population this count rarely exceeds 27 glutamines. A sequence of 36 or more glutamines, however, results in the production of form of HTT which has different characteristics, this form is called mutant HTT or more commonly mHTT. The presence of mHTT increases the rate neuron decay in select types of neuron and areas of the brain. Generally, the number of CAG repeats is related to how much this process is affected, and correlates with age at onset and the rate of progression of symptoms. For example, 36–39 repeats result in much later onset and slower progression of symptoms than the mean, such that some individuals may die of other causes before they even manifest symptoms of Huntington disease, this is termed "reduced penetrance". No case of HD has been diagnosed with a count of less than 36.
Inital studies show carriers of the gene may have better than average immune systems, with higher levels of ] and tumor suppressor protein p53.
Inheritance
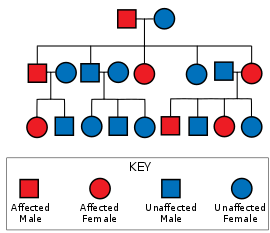
Huntington's disease is inherited autosomal dominantly, meaning that an affected individual typically inherits a copy of the gene with an expanded trinucleotide repeat (the mutant allele) from an affected parent. In this type of inheritance pattern, each offspring of an affected individual has a 50% chance of inheriting the mutant allele and therefore being affected with the disorder (see figure). It is extremely rare for Huntington's disease to be caused by a de novo mutation, however, the inheritance of HD is more complex due to potential dynamic mutations, where DNA replication does not produce an exact copy of the trinucleotide repeat. This can cause the number of repeats to change in successive generations, such that an unaffected parent with an "intermediate" number of repeats (28-35), or "reduced penetrance" (36-39), may pass on a copy of the gene with an increase in the number of repeats that produces fully penetrant HD. Maternally inherited alleles are usually of a similar repeat length, whereas paternally inherited ones seem to have a higher chance of increasing in length. Increases in the number of repeats (and hence earlier age of onset and severity of disease) in successive generations is known as genetic anticipation.
Individuals homozygous for mutant HTT alleles are very rare, except in large consanguineous families. While HD seemed to be the first disease for which homozygotes did not differ in clinical expression or course from typical heterozygotes, more recent analysis suggests that homozygosity affects the phenotype and the rate of disease progression but does not alter the age of onset.
Mechanism
See also: Huntingtin proteinLike all proteins, Htt and mHtt are translated, perform or affect biological functions, and are then marked by Ubiquitin to be degraded by Proteasomes, but their exact roles are unknown. Research has focused on identifying the functioning of Htt, how mHtt differs or interferes with it, including its influence in programmed cell death, and the proteopathy of remnants of mHtt left after degradation.
Function
Although the function of Huntingtin is unclear, some hypotheses have been drawn from numerous observations. In mouse models HTT is essential for development and survival. In "knockin" mice, the extended CAG repeat portion of the gene is all that is needed to cause disease. The protein has no sequence homology with other proteins and is highly expressed in neurons and testes. Experiments have shown HTT acts as a transcription factor in upregulating the expression of Brain Derived Neurotrophic Factor (BDNF), a protein which protects neurons and regulates the neurogenesis of new ones, whereas mHTT suppresses this transcription regulatory function of Huntingtin and hence underexpression of BDNF, which leads to progressive atrophy of select areas of the brain.From immunohistochemistry, electron microscopy, subcellular fractionation studies of the molecule, it has been found that Huntingtin is primarily associated with vesicles and microtubules..These indicate a functional role in cytoskeletal anchoring or transport of mitochondria. The HTT protein is involved in vesicle trafficking as it interacts with HIT1, a clathrin binding protein, to mediate endocytosis, the absorption of materials into a cell.
Degradation
In the first step of degradation, both Htt and mHtt are cleaved by caspase-3, which removes the protein's amino end (the N-terminal). Caspase-2 then further breaks down the amino terminal fragment (which includes the CAG repeat) of Htt, but cannot process all of the mHtt protein. In transgenic mice, the caspase-6 enzyme has been shown to be involved in cleaving the Htt protein, as mice made resistant to this enzyme did not suffer neurodegeneration.The uncleaved pieces of mHTT, left in the cell, called N-fragments, and are able to affect genetic transcription. Specifically, mHtt binds with TAFII130, a coactivator to CREB dependent transcription. The mHTT protein also interacts with the transcription factor protein SP1, preventing it from binding to DNA.
Neuronal intranuclear inclusions, containing the proteins huntingtin and ubiquitin have been found in both humans and mice with HD, and are most prevalent in cortical pyramidal neurons, less so in striatal medium-sized spiny neurons and almost entirely absent in most other regions. These inclusions consist mainly of the amino terminal end of mHtt (CAG repeat), and are found in both the cytoplasm and nucleus of neurons, however their presence does not correlate with cell death. Thus, mHtt acts in the nucleus but does not cause apoptosis through protein aggregation.
Pathophysiology
HD causes astrogliosis and loss of medium spiny neurons Areas of the brain are affected according to their structure and the types of neurons they contain, reducing in size as they cumulatively lose cells. The areas affected are mainly in the striatum, but also the frontal and temporal cortices. The striatum's subthalamic nuclei send control signals to the globus pallidus, which initiates and modulates motion. The weaker signals from subthalamic nuclei thus cause reduced initiation and modulation of movement, resulting in the characteristic movements of the disorder.
Although studies are mainly focused on physiopathology of the brain, a few look at how Huntington's disease appears to affect other physiological functions. One study in humans highlighted a systemic, early hypermetabolic state and a lower level of branched chain amino acids in the plasma as the disease progresses, which is hypothesised to be caused by the brain's use of these.
Diagnosis
Further information: Genetic testingTo determine whether initial symptoms are evident, a physical and/or psychological examination is required. Abnormal movements are often the symptoms that cause initial impetus to seek medical consultation and lead to diagnosis; however, the disease may begin with cognitive or pyschiatric symptoms, which are not always recognized until they develop further. Pre-symptomatic testing is possible using a blood test which counts the numbers of CAG repeats in each of the HTT alleles, although a positive result is not considered a diagnosis, since it may be obtained decades before onset of symptoms. A negative blood test means that the individual does not carry the expanded copy of the gene. A full pathological diagnosis can only be established by a neurological examination's findings and/or demonstration of cell loss in the areas affected by HD, supported by a cranial CT or MRI scan findings.
A pre-symptomatic test is a life-changing event and a very personal decision. The personal ramifications to an at-risk individual and lack of cure for the disease necessitate several counseling sessions to ensure that they are prepared for either result before it is given. Unless a person under eighteen years of age is considered to be Gillick competent, testing is not offered by many institutions unless they show significant symptoms or are sexually active. Many organisations and lay groups strongly endorse these restrictions in their testing protocol. Embryonic screening is also possible, giving affected or at-risk individuals the option of ensuring their children will not inherit the disease. If abortion is acceptable to the individual it is possible to test an embryo in the womb (prenatal diagnosis) but it can be ensured to have an unaffected child by utilising in vitro fertilisation and testing before implantation.
Management
There is no treatment to fully arrest the progression of the disease, but symptoms can be reduced or alleviated through the use of medication, rehabilitation methods, nutrition management and general care methods.
Pharmacological
No evidence has given a general Pharmacological treatment, so medications specific to certain symptoms are used. Tetrabenazine may be used to reduce chorea and on August 15th 2008, became the first FDA approved medication . Treatments for cognitive and psychological symptoms include antidepressants and sedatives, and low doses of antipsychotics.
Rehabilitation
Although there are relatively few studies of rehabilitation for HD, its general effectiveness when conducted by a team of specialists has been clearly demonstrated in other pathologies such as stroke, or head trauma. As for any patient with neurologic deficits, a multidisciplinary approach is key to limiting and overcoming disability. There is partial evidence for the usefulness of physiotherapy and speech therapy but most studies on the subject have been of low quality and further evidence is needed for health authorities to endorse them.
Nutrition
Nutrition is an important part of treatment; most people in the later stages of the disease require two to three times more calories than average to maintain body weight. Thickening agent can be added to drinks as swallowing becomes more difficult, as thicker fluids are easier and safer to swallow. The option of using a stomach PEG is available when eating becomes too hazardous or uncomfortable; this greatly reduces the chances of aspiration of food, which can lead to Aspiration pneumonia one of the most common causes of death of the condition, and increases the amount of nutrients and calories that can be ingested, aiding the body's natural defenses.
Prognosis
The age of onset decreases, and the rate of progression of symptoms increase, with the number of CAG repeats. Individuals with greater than approximately 60 CAG repeats often develop juvenile Huntington's disease. There is a variation in age of onset for any given CAG repeat length, particularly within the intermediate range (40–50 CAGs). For example, a repeat length of 40 CAGs leads to an onset ranging from 40 to 70 years of age in one study. This variation means that, although algorithms have been proposed for predicting the age of onset, in practice, it can not be predicted confidently.
Following the onset of characteristic manifestations of the disorder, life expectancy is generally 15–20 additional years. Mortality is not caused by Huntington’s disease directly, but by associated complications; these include pneumonia (which causes one third of fatalities), heart failure (although heart disease, cerebrovascular disease and atherosclerosis show no increase), choking, malnutrition and physical injury. Suicide is an associated risk, with increased suicide rates of up to 7.3 percent, and attempted suicides of up to 27 percent.
Epidemiology
As HD is autosomal dominant, and does not usually affect reproduction, areas of increased prevalence occur according to historical migration of carriers, some of which can be traced back thousands of years using genetic haplotypes. New mutations are less than 10 percent of HD carriers, but account for the less localised occurrences, and origins, of the disorder. Since the discovery of a genetic test that can also be used pre-symptomatically, estimates of the incidence of the disorder are likely to increase. Without the genetic test, only individuals displaying physical symptoms or neurologically examined cases were diagnosed, excluding any who died of other causes before symptoms or diagnosis occurred. These cases can now be included in statistics as the test becomes more widely available and estimates have shown the incidence of HD could be two to three times higher when these results are included.
The prevalence varies greatly according to geographical location, both by ethnicity and local migration; The highest occurrence is in peoples of Western Europe descent, averaging between 3 to 7 per 100,000 people, but is relatively lower in the rest of the world, e.g. 1 per 1,000,000 of Asian and African descent. Some localised areas have a much higher prevalence than their geographical average, for example the isolated populations of the Lake Maracaibo region of Venezuela (where the marker for the gene was discovered), have an extremely high prevalence of up to 700 per 100,000, leading to the conclusion that one of their initial founders must have been a carrier of the gene. This is known as the local founder effect.
About 7% of HD cases occur in people under the age of 20 years. This is referred to as juvenile HD, akinetic-rigid, or Westphal variant HD.
History
In the first part of the twentieth century and earlier, many people with HD were misdiagnosed as suffering from alcoholism or manic depression. Previously mortality due to starvation or dehydration was a major risk.
- c. 300: Along with other conditions with abnormal movements, it may have been referred to as St Vitus' dance. St Vitus is the patron saint of epileptics who was martyred in 303.
- Middle Ages: People with the condition were probably persecuted as being witches or as being possessed by spirits, and were shunned, exiled or worse. Some speculate that the "witches" in the Salem Witch Trials in 1692 had HD.
- 1860: One of the early medical descriptions of HD was made in 1860 by a Norwegian district physician, Johan Christian Lund. He noted that in Setesdalen, a remote and rather secluded area, there was a high prevalence of dementia associated with a pattern of jerking movement disorders that tended to run in families. This is the reason for the disease being commonly referred to as Setesdalsrykkja (Setesdalen=the location, rykkja=jerking movements) in Norwegian.
- 1872: George Huntington was the third generation of a family medical practice in Long Island. With their combined experience of several generations of a family with the same symptoms, he realised their conditions were linked and set about describing it. A year after leaving medical school, in 1872, he presented his accurate definition of the disease to a medical society in Middleport, Ohio.
- c. 1923: Smith Ely Jelliffe (1866–1945) and Frederick Tilney (1875–1938) began analyzing the history of HD sufferers in New England.
- 1932: P. R. Vessie expanded Jelliffe and Tilney's work, tracing about a thousand people with HD back to two brothers and their families who left Bures in Suffolk, England bound for Boston, MA in 1630.
- 1979: The US-Venezuela Huntington's Disease Collaborative Research Project began an extensive study which gave the basis for the gene to be discovered. This was conducted in the small, isolated Venezuelan fishing villages of Barranquitas and Lagunetas, where families have a high presence of the disease.
- 1983: James Gusella, David Housman, P. Michael Conneally, Nancy Wexler, and their colleagues find the general location of the gene, using DNA marking methods for the first time—an important first step toward the Human Genome Project.
- 1992: Anita Harding, et al. find that trinucleotide repeats affect disease severity
- 1993: The Huntington's Disease Collaborative Research Group isolates the precise gene at 4p16.3.
- 1996: A transgenic mouse (the R6 line) was created that could be made to exhibit HD, greatly advancing how much experimentation can be achieved.
- 1997: DiFiglia M, Sapp E, Chase KO, et al., discover that mHtt fragments misfold, leading to the formation of nuclear inclusions.
- The full record of research is extensive.
Society and culture
See also: List of Huntington's disease media depictionsAs public awareness has increased, HD has been depicted increasingly in numerous books, films and TV series. Early works were Arlo Guthrie's 1969 film Alice's Restaurant and Jacqueline Susann's 1966 American novel Valley of the Dolls; more recent references were made in ER, Private Practice, Everwood, All Saints, and House. Many support organizations hold an annual HD awareness event, and some have been endorsed by their respective governments, for example, June 6th is designated "National Huntington's Disease Awareness Day" by the USA senate, and the UK HDA holds an awareness campaign in the third week of June.
Social impact
The development of an accurate diagnostic test for Huntington's disease has caused social, legal, and ethical concerns over access and use of a person's results There have been guidelines created by the IHA and WFN for the use of genetic testing in HD, but many governments and institutions, although agreeing not to genetically descriminate against an individual, have not reached a consensus. As for the individual, the decision to undergo a presymptomatic test, thereby learning whether they will inherit the disorder, is a complicated one. Genetic counseling is designed to provide information, advice and support for a person to decide, and then throughout all stages of the testing process, if so chosen.
One of the key issues is the level of anxiety that an individual experiences about "not knowing" whether they will some day develop HD, compared to the psychological impact that might occur if the test indicates that one will eventually succumb to the disease. In contrast individuals found to have inherited the normal allele may experience Survivor guilt with regard to their affected family members. Some individuals choose not to undergo testing due to concerns about prejudice in areas such as insurability, employment and financial matters. An important ethical consideration is the testing of asymptomatic minors. As with other genetic conditions with later onset for which no treatment is available, it is ethically questionable to perform presymptomatic testing for a child or individual who has not made a mature, informed decision for themselves. Such information may cause psychological harm, jeapordize that individual's employment or ability to obtain health and life insurance. For this reason, presymptomatic testing in minors is a controversial area, with arguments in favor of permitting testing only when the individual is mature enough to provide assent versus minimizing medical paternalism in favor of parental preferences. The balance of opinion would change drastically if an effective treatment was discovered.
Genetic testing may infer information about relatives who do not want it. Testing a descendant of a undiagnosed parent has implications to other family members, since a positive result automatically reveals the parent as carrying the affected gene, and siblings (and especially identical twins) as being 'at risk' of also inheriting it. This emphasizes the importance of disclosure, as individuals have to decide when and how to reveal the information to their children and other family members. For those at risk, or known to carry a mutant allele, there can be the consideration of prenatal genetic testing and preimplantation genetic diagnosis in order to ensure that the disorder is not passed on.
Huntington's disease has tested society's ethics in various ways. HD was one of the targets of the eugenics movement, in which American scientist Charles Davenport proposed in 1910 that compulsory sterilization and immigration control be used for people with certain diseases, including HD. Since the development of genetic testing for HD, financial institutions and businesses are also faced with the question of whether to use results when assessing an individual, e.g. for life insurance or employment. Some countries' organizations have already agreed not to use this information. Recent passage of the Genetic Information Nondiscrimination Act (GINA) in the United States prevents health insurance companies from denying coverage or charging higher premiums for individuals with predisposition to genetic disease.
Organizations
- 1967: Woody Guthrie's wife, Marjorie, helped found the Committee to Combat Huntington's Disease, after his death from HD complications. This eventually became the Huntington's Disease Society of America. Since then, lay organizations have been formed in many countries around the world.
- 1968: After experiencing HD in his wife's family Dr. Milton Wexler was inspired to start the Hereditary Disease Foundation (HDF). Professor Nancy S. Wexler, Dr. Wexler's daughter, was in the research team in Venezuela and is now president of the HDF.
- 1974: The first international meeting took place when the founders of the Canadian HD Society (Ralph Walker) and of the British HD Society (Mauveen Jones) attended the annual meeting of the American HD Society.
- 1977: Second meeting organized by the Dutch Huntington Society the "Vereniging van Huntington", representatives of six countries were present.
- 1979: International Huntington Association (IHA) formed during international meeting in Oxford, England organized by HDA of England.
- 1981–2001: Biennial meetings held by IHA which became the World Congress on HD.
- 2003: The first World Congress on Huntington's Disease was held in Toronto. This is a biennial meeting for associations and researchers to share ideas and research, which is held on odd-number years. The Euro-HD Network was started as part of the Huntington Project, funded by the High-Q Foundation.
Research directions
Appropriate animal models are critical for understanding the fundamental mechanisms causing the disease and for supporting the early stages of drug development. Neurochemically induced mice or monkeys were first available, but they did not mimic the progressive features of the disease. After the HD gene was discovered, transgenic animals exhibiting HD were generated by inserting a CAG repeat expansion into their genome, these were of mice (strain R6/2),), Drosophila fruit flies, and more recently monkeys. Expression without insertion of a CAG repeat in nematode worms also produced a valuable model.
Intrabody therapy
Genetically engineered antibody fragments called intrabodies have shown therapeutic results, by inhibiting mHtt aggregation, in drosophila models. This was achieved using an intrabody called C4 sFv, a single chain variable fragment which binds to the end of mHtt within a cell. This therapy prevented larval and pupal mortality (without therapy 77% died) and delayed neurodegeneration in the adult, significantly increasing their lifespan. Intrabody therapy shows promise as a tool for drug discovery, and as a potential therapy for HD and other neurodegenerative disorders caused by protein mis-folding or abnormal protein interactions.
Gene silencing
As HD has been conclusively linked to a single gene, gene silencing is potentially possible. Researchers have investigated using gene knockdown of mHTT as a potential treatment. Using a mouse model, siRNA therapy achieved a 60 percent reduction in expression of the mHTT and progression of the disease was stalled. However, this study used the human form of the mHTT protein in the mouse, and was therefore only able to directly target the mHTT, leaving endogenous, wild-type mouse Htt gene expression unaffected. From a practical standpoint, it would be difficult in humans to use siRNA to target the mutant form while leaving the normal copy unaffected. The precise function of HTT is unknown, but in mice, complete deletion of the Htt gene is lethal. Thus, using RNA interference to treat HD could have unexpected effects unless knock-down of the normal HTT protein can be avoided. Other issues include problems delivering the siRNA to the appropriate target tissue, off-target effects of siRNA, and toxicity from shRNA over-expression. Another study showed that mouse models already in late stages of the disease recovered motor function after expression of mHTT was stopped.
Stem cell implants
Main article: Stem cell treatmentsStem cell therapy is the replacement of damaged neurons by transplantation of stem cells ( or possibly neural stem cells - a type of somatic (adult) stem cell ) into affected regions of the brain. Hypothetically, embryonic stem cells can be differentiated into neuronal precursors in vitro, and transplanted into damaged areas of the brain to generate replacement neurons, if enough damaged neurons can be replaced and develop the correct synaptic connectivity, symptoms could be alleviated. This treatment would not prevent further neuronal damage, so it would have to be an ongoing treatment. Experiments have yielded some positive results in animal models, but remains highly speculative and has not been tested in clinical trials.
Pharmacological
As of August 2008, several trials of various compounds are in development or ongoing, a few at the point of testing on larger numbers of people, known as phase III of clinical trials. Substances that have shown promise in initial experiments include dopamine receptor blockers, select dopamine antagonists, such as tetrabenazine, creatine, CoQ10, the antibiotic Minocycline, antioxidant-containing foods and nutrients, and antidepressants, including selective serotonin reuptake inhibitors (SSRIs) such as sertraline, fluoxetine, and paroxetine.
References
- ^ Gillian Bates, Peter Harper, and Lesley Jones (2002). Huntington's Disease - Third Edition. Oxford: Oxford University Press. ISBN 0-19-851060-8.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Bonelli RM, Wenning GK (2006). "Pharmacological management of Huntington's disease: An evidence-based review". Curr. Pharm. Des. 12 (21): 2701–20. PMID 16842168. Cite error: The named reference "pmid16842168" was defined multiple times with different content (see the help page).
- ^ NCBI OMIM. "Huntington's Disease". Retrieved 2008-05-22.
- ^ Nance MA, Myers RH (2001). "Juvenile onset Huntington's disease--clinical and research perspectives". Ment Retard Dev Disabil Res Rev. 7 (3): 153–7. doi:10.1002/mrdd.1022. PMID 11553930.
- ^ Kieburtz K, MacDonald M, Shih C; et al. (1994). "Trinucleotide repeat length and progression of illness in Huntington's disease". J. Med. Genet. 31 (11): 872–4. PMID 7853373.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Imarisio S, Carmichael J, Korolchuk V; et al. (2008). "Huntington's disease: from pathology and genetics to potential therapies". Biochem. J. 412 (2): 191–209. doi:10.1042/BJ20071619. PMID 18466116.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Huntington, G. (1872-04-13). "On Chorea". Medical and Surgical Reporter of Philadelphia. 26 (15): 317–321.
- ^ Gusella JF, Wexler NS, Conneally PM; et al. (1983). "A polymorphic DNA marker genetically linked to Huntington's disease". Nature. 306 (5940): 234–8. doi:10.1038/306234a0. PMID 6316146.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - "A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group". Cell. 72 (6): 971–83. 1993. PMID 8458085.
{{cite journal}}: Unknown parameter|month=ignored (help) - Walker FO (2007). "Huntington's disease". Lancet. 369 (9557): 218–28. doi:10.1016/S0140-6736(07)60111-1. PMID 17240289.
{{cite journal}}: Unknown parameter|month=ignored (help) - Gaba AM, Zhang K, Marder K, Moskowitz CB, Werner P, Boozer CN (2005). "Energy balance in early-stage Huntington disease". Am. J. Clin. Nutr. 81 (6): 1335–41. PMID 15941884.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - "Booklet by the Huntington Society of Canada" (PDF). Caregiver's Handbook for Advanced-Stage Huntington Disease. HD Society of Canada. 2007-04-11. Retrieved 2008-08-10.
- Morton AJ, Wood NI, Hastings MH; et al. (2005). "Disintegration of the sleep-wake cycle and circadian timing in Huntington's disease". J. Neurosci. 25 (1): 157–63. doi:10.1523/JNEUROSCI.3842-04.2005. PMID 15634777.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Arnulf I, Nielsen J, Lohmann E; et al. (2008). "Rapid eye movement sleep disturbances in Huntington disease". Arch Neurol. 65 (4): 482–488. PMID 18413470.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - "Cognitive Impairment: Symptoms to Diagnosis Toward Optimized Practice" (PDF). Alberta Medical Association. 2007. Retrieved 2008-08-10.
- Johnson SA, Stout JC, Solomon AC; et al. (2007). "Beyond disgust: Impaired recognition of negative emotions prior to diagnosis in Huntington's disease". Brain. 130 (Pt 7): 1732–44. doi:10.1093/brain/awm107. PMID 17584778.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Sandyk R (1992). "L-tryptophan in neuropsychiatric disorders: A review". Int. J. Neurosci. 67 (1–4): 127–44. PMID 1305630.
- van Duijn E, Kingma EM, van der Mast RC (2007). "Psychopathology in verified Huntington's disease gene carriers". J Neuropsychiatry Clin Neurosci. 19 (4): 441–8. doi:10.1176/appi.neuropsych.19.4.441. PMID 18070848.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Katsuno M, Banno H, Suzuki K; et al. (2008). "Molecular genetics and biomarkers of polyglutamine diseases". Curr. Mol. Med. 8 (3): 221–34. PMID 18473821.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Chong SS, Almqvist E, Telenius H; et al. (1997). "Contribution of DNA sequence and CAG size to mutation frequencies of intermediate alleles for Huntington disease: Evidence from single sperm analyses" (PDF). Hum. Mol. Genet. 6 (2): 301–9. PMID 9063751.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Björkqvist M, Wild EJ, Thiele J; et al. (2008). "A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington's disease". J. Exp. Med. 205 (8): 1869–77. doi:10.1084/jem.20080178. PMID 18625748.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Eskenazi BR,Wilson-Rich NS, Starks PT (2007). Med. Hypotheses. 69 (6): 1183–9. doi:10.1016/j.mehy.2007.02.046. PMID 17689877.
{{cite journal}}: Missing or empty|title=(help); Text "A Darwinian approach to Huntington's disease:subtle health benefits of a neurological disorder" ignored (help)CS1 maint: multiple names: authors list (link) - Myers; et al. (1993). "De novo expansion of a (CAG)n repeat in sporadic Huntington's disease". Nature Genetics. 5: 168–173. doi:10.1038/ng1093-168.
{{cite journal}}: Explicit use of et al. in:|author=(help) - Sánchez A, Milà M, Castellví-Bel S, Rosich M, Jiménez D, Badenas C, Estivill X. (1997). "Maternal transmission in sporadic Huntington's disease". J Neurol Neurosurg Psychiatry. 62 (5): 535–537. PMC 486880. PMID 9153618.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - RM Ridley, CD Frith, TJ Crow and PM Conneally (1988). "Anticipation in Huntington's disease is inherited through the male line but may originate in the female". Journal of Medical Genetics. 25: 589–595. PMID 2972838.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Wexler NS, Young AB, Tanzi RE; et al. (1987). "Homozygotes for Huntington's disease". Nature. 326 (6109): 194–197. doi:10.1038/326194a0. PMID 2881213.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Squitieri F, Gellera C, Cannella M, Mariotti C, Cislaghi G, Rubinsztein DC, Almqvist EW, Turner D, Bachoud-Lévi AC, Simpson SA, Delatycki M, Maglione V, Hayden MR, Donato SD (2003). "Homozygosity for CAG mutation in Huntington disease is associated with a more severe clinical course". Brain. 126 (4): 946–955. doi:10.1093/brain/awg077. PMID 12615650.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Nasir J, Floresco S; et al. (1995). "Targeted disruption of the Huntington's disease gene results in embryonic lethality and behavioral and morphological changes in heterozygotes". Cell. 81: 811–823. doi:10.1016/0092-8674(95)90542-1.
{{cite journal}}: Explicit use of et al. in:|author=(help) - Murphy KP, Carter RJ, Lione LA; et al. (2000). "Abnormal synaptic plasticity and impaired spatial cognition in mice transgenic for exon 1 of the human Huntington's disease mutation". J. Neurosci. 20 (13): 5115–23. PMID 10864968.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Cattaneo E, Zuccato C, Tartari M (2005 Dec). "Normal huntingtin function: an alternative approach to Huntington's disease". Nature Reviews Neuroscience. 6: 919–930. doi:10.1038/nrn1806.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: multiple names: authors list (link) - Zuccato C, Ciammola A, Rigamonti D, Leavitt BR, Goffredo D; et al. (2001 Jul 20). "Loss of huntingtin-mediated BDNF gene transcription in Huntington's disease". Science. 293 (5529): 445–6. doi:10.1126/science.1063429. PMID 11463904.
{{cite journal}}: Check date values in:|date=(help); Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Canals JM, Pineda JR, Torres-Peraza JF; et al. (2004). "Brain-derived neurotrophic factor regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington's disease". J. Neurosci. 24 (35): 7727–39. doi:10.1523/JNEUROSCI.1197-04.2004. PMID 15342740.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Sawa A, Nagata E, Sutcliffe S; et al. (2005). "Huntingtin is cleaved by caspases in the cytoplasm and translocated to the nucleus via perinuclear sites in Huntington's disease patient lymphoblasts". Neurobiol. Dis. 20 (2): 267–74. doi:10.1016/j.nbd.2005.02.013. PMID 15890517.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Strand AD, Baquet ZC, Aragaki AK; et al. (2007). "Expression profiling of Huntington's disease models suggests that brain-derived neurotrophic factor depletion plays a major role in striatal degeneration". J. Neurosci. 27 (43): 11758–68. doi:10.1523/JNEUROSCI.2461-07.2007. PMID 17959817.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Hoffner G, Kahlem P, Djian P (2002). "Perinuclear localization of huntingtin as a consequence of its binding to microtubules through an interaction with β-tubulin: relevance to Huntington's disease". J Cell Sci. 115: 941–948.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - DiFiglia M; et al. (1995). "Huntingtin is a cytoplasmic protein associated with vesicles in human and rat brain neurons". Neuron. 14: 1075–1081. doi:10.1016/0896-6273(95)90346-1.
{{cite journal}}: Explicit use of et al. in:|author=(help) - Velier J, Kim M, Schwarz C; et al. (1998). "Wild-type and mutant huntingtins function in vesicle trafficking in the secretory and endocytic pathways". Exp. Neurol. 152 (1): 34–40. doi:10.1006/exnr.1998.6832. PMID 9682010.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Waelter S, Scherzinger E, Hasenbank R; et al. (2001). "The huntingtin interacting protein HIP1 is a clathrin and alpha-adaptin-binding protein involved in receptor-mediated endocytosis". Hum. Mol. Genet. 10 (17): 1807–17. doi:10.1093/hmg/10.17.1807. PMID 11532990.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Kim YJ, Yi Y, Sapp E; et al. (2001). "Caspase 3-cleaved N-terminal fragments of wild-type and mutant huntingtin are present in normal and Huntington's disease brains, associate with membranes, and undergo calpain-dependent proteolysis". Proc. Natl. Acad. Sci. U.S.A. 98 (22): 12784–9. doi:10.1073/pnas.221451398. PMID 11675509.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Hermel E, Gafni J, Propp SS; et al. (2004). "Specific caspase interactions and amplification are involved in selective neuronal vulnerability in Huntington's disease". Cell Death Differ. 11 (4): 424–38. doi:10.1038/sj.cdd.4401358. PMID 14713958.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Graham RK, Deng Y, Slow EJ; et al. (2006). "Cleavage at the caspase-6 site is required for neuronal dysfunction and degeneration due to mutant huntingtin". Cell. 125 (6): 1179–91. doi:10.1016/j.cell.2006.04.026. PMID 16777606.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Freiman RN, Tjian R (2002). "Neurodegeneration. A glutamine-rich trail leads to transcription factors". Science. 296 (5576): 2149–50. doi:10.1126/science.1073845. PMID 12077389.
- Bae BI, Xu H, Igarashi S; et al. (2005). "p53 mediates cellular dysfunction and behavioral abnormalities in Huntington's disease". Neuron. 47 (1): 29–41. doi:10.1016/j.neuron.2005.06.005. PMID 15996546.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Dunah AW, Jeong H, Griffin A; et al. (2002). "Sp1 and TAFII130 transcriptional activity disrupted in early Huntington's disease". Science. 296 (5576): 2238–43. doi:10.1126/science.1072613. PMID 11988536.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ DiFiglia M, Sapp E, Chase KO; et al. (1997). "Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain". Science. 277 (5334): 1990–3. doi:10.1126/science.277.5334.1990. PMID 9302293.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Davies SW, Turmaine M, Cozens BA; et al. (1997). "Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation". Cell. 90 (3): 537–48. doi:10.1016/S0092-8674(00)80513-9. PMID 9267033.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Gutekunst CA, Li SH, Yi H; et al. (1999). "Nuclear and neuropil aggregates in Huntington's disease: Relationship to neuropathology". J. Neurosci. 19 (7): 2522–34. PMID 10087066.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Sieradzan KA, Mechan AO, Jones L; et al. (1999). "Huntington's disease intranuclear inclusions contain truncated, ubiquitinated huntingtin protein". Exp. Neurol. 156 (1): 92–9. doi:10.1006/exnr.1998.7005. PMID 10192780.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Cooper JK, Schilling G, Peters MF; et al. (1998). "Truncated N-terminal fragments of huntingtin with expanded glutamine repeats form nuclear and cytoplasmic aggregates in cell culture". Hum. Mol. Genet. 7 (5): 783–90. doi:10.1093/hmg/7.5.783. PMID 9536081.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Fusco FR, Chen Q, Lamoreaux WJ; et al. (1999). "Cellular localization of huntingtin in striatal and cortical neurons in rats: Lack of correlation with neuronal vulnerability in Huntington's disease". J. Neurosci. 19 (4): 1189–202. PMID 9952397.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Saudou F, Finkbeiner S, Devys D, Greenberg ME (1998). "Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions". Cell. 95 (1): 55–66. doi:10.1016/S0092-8674(00)81782-1. PMID 9778247.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Lobsiger CS, Cleveland DW (2007). "Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease". Nat. Neurosci. 10 (11): 1355–60. doi:10.1038/nn1988. PMID 17965655.
{{cite journal}}: Unknown parameter|month=ignored (help) - Purves D, Augustine GA, Fitzpatrick D, Hall W, LaMantia A-S, McNamara JO, Williams SM. "Modulation of Movement by the Basal Ganglia - Circuits within the Basal Ganglia System". In Dale Purves (ed.). Neuroscience (2nd edition ed.). Sunderland, MA: Sinauer Associates. ISBN 0-87893-742-0.
{{cite book}}:|edition=has extra text (help); External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help)CS1 maint: multiple names: authors list (link) - Estrada Sánchez AM, Mejía-Toiber J, Massieu L (2008). "Excitotoxic neuronal death and the pathogenesis of Huntington's disease". Arch. Med. Res. 39 (3): 265–76. doi:10.1016/j.arcmed.2007.11.011. PMID 18279698.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Purves D, Augustine GA, Fitzpatrick D, Hall W, LaMantia A-S, McNamara JO, Williams SM. "Modulation of Movement by the Basal Ganglia - Box A. Huntington's Disease". In Dale Purves (ed.). Neuroscience (2nd edition ed.). Sunderland, MA: Sinauer Associates. ISBN 0-87893-742-0.
{{cite book}}:|edition=has extra text (help); External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help)CS1 maint: multiple names: authors list (link) - Crossman AR (2000). "Functional anatomy of movement disorders" (PDF). J. Anat. 196 ( Pt 4): 519–25. doi:10.1046/j.1469-7580.2000.19640519.x. PMC 1468094. PMID 10923984.
{{cite journal}}: Unknown parameter|month=ignored (help) - Mochel F, Charles P, Seguin F, Barritault J, Coussieu C, Perin L, Le Bouc Y, Gervais C, Carcelain G, Vassault A, Feingold J, Rabier D, Durr A (2007). "Early energy deficit in Huntington disease: Identification of a plasma biomarker traceable during disease progression". PLoS ONE. 2 (7). PMID 17653274.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Myers RH (2004). "Huntington's disease genetics". NeuroRx. 1 (2): 255–62. PMC 534940. PMID 15717026.
{{cite journal}}: Unknown parameter|month=ignored (help) - Stober T, Wussow W, Schimrigk K (1984). "Bicaudate diameter--the most specific and simple CT parameter in the diagnosis of Huntington's disease". Neuroradiology. 26 (1): 25–8. doi:10.1007/BF00328198. PMID 6234475.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Burson CM, Markey KR (2001). "Genetic counseling issues in predictive genetic testing for familial adult-onset neurologic diseases". Semin Pediatr Neurol. 8 (3): 177–86. PMID 11575847.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Borry P, Goffin T, Nys H, Dierickx K (2008). "Predictive genetic testing in minors for adult-onset genetic diseases". Mt. Sinai J. Med. 75 (3): 287–296. doi:10.1002/msj.20038. PMID 18704981.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Kuliev A, Verlinsky Y (2005). "Preimplantation diagnosis: A realistic option for assisted reproduction and genetic practice". Curr. Opin. Obstet. Gynecol. 17 (2): 179–83. PMID 15758612.
{{cite journal}}: Unknown parameter|month=ignored (help) - "UK NHS guide to Huntington's medications". NHS. Retrieved 2008-08-10.
- "Tetrabenazine as antichorea therapy in Huntington disease: a randomized controlled trial". Neurology. 66 (3): 366–72. 2006. doi:10.1212/01.wnl.0000198586.85250.13. PMID 16476934.
{{cite journal}}: Unknown parameter|month=ignored (help) - "Organised inpatient (stroke unit) care for stroke. Stroke Unit Trialists' Collaboration". Cochrane Database Syst Rev (2): CD000197. 2000. doi:10.1002/14651858.CD000197. PMID 10796318.
- Turner-Stokes L, Disler PB, Nair A, Wade DT (2005). "Multi-disciplinary rehabilitation for acquired brain injury in adults of working age". Cochrane Database Syst Rev (3): CD004170. doi:10.1002/14651858.CD004170.pub2. PMID 16034923.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Zinzi P, Salmaso D, De Grandis R; et al. (2007). "Effects of an intensive rehabilitation programme on patients with Huntington's disease: a pilot study". Clin Rehabil. 21 (7): 603–13. doi:10.1177/0269215507075495. PMID 17702702.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Bilney B, Morris ME, Perry A (2003). "Effectiveness of physiotherapy, occupational therapy, and speech pathology for people with Huntington's disease: a systematic review". Neurorehabil Neural Repair. 17 (1): 12–24. PMID 12645441.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Gaba, Anna. "Family Guide Series - Nutrition and Huntington's Disease". Huntington's Disease Society of America Publications. Retrieved 2008-04-02.
- Panagiotakis PH, DiSario JA, Hilden K, Ogara M, Fang JC (2008). "DPEJ tube placement prevents aspiration pneumonia in high-risk patients". Nutr Clin Pract. 23 (2): 172–5. doi:10.1177/0884533608314537. PMID 18390785.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Harper PS (1999). "Huntington's disease: A clinical, genetic and molecular model for polyglutamine repeat disorders". Philos. Trans. R. Soc. Lond., B, Biol. Sci. 354 (1386): 957–61. doi:10.1098/rstb.1999.0446. PMID 10434293.
- Andrew SE, Goldberg YP, Kremer B; et al. (1993). "The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington's disease". Nat. Genet. 4 (4): 398–403. doi:10.1038/ng0893-398. PMID 8401589.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Rubinsztein DC, Leggo J, Chiano M; et al. (1997). "Genotypes at the GluR6 kainate receptor locus are associated with variation in the age of onset of Huntington disease". Proc. Natl. Acad. Sci. U.S.A. 94 (8): 3872–6. doi:10.1073/pnas.94.8.3872. PMID 9108071.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Adams P, Falek A, Arnold J (1988). "Huntington disease in Georgia: Age at onset". Am. J. Hum. Genet. 43 (5): 695–704. PMID 2973230.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Roos RA, Hermans J, Vegter-van der Vlis M; et al. (1993). "Duration of illness in Huntington's disease is not related to age at onset". J. Neurol. Neurosurg. Psychiatr. 56 (1): 98–100. PMID 8429330.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Lanska DJ, Lanska MJ, Lavine L, Schoenberg BS (1988). "Conditions associated with Huntington's disease at death. A case-control study". Arch. Neurol. 45 (8): 878–80. PMID 2969233.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Di Maio L, Squitieri F, Napolitano G; et al. (1993). "Suicide risk in Huntington's disease". J. Med. Genet. 30 (4): 293–5. PMID 8487273.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Schoenfeld M, Myers RH, Cupples LA; et al. (1984). "Increased rate of suicide among patients with Huntington's disease". J. Neurol. Neurosurg. Psychiatr. 47 (12): 1283–7. PMID 6239910.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Squitieri F, Andrew SE, Goldberg YP; et al. (1994). "DNA haplotype analysis of Huntington disease reveals clues to the origins and mechanisms of CAG expansion and reasons for geographic variations of prevalence". Hum. Mol. Genet. 3 (12): 2103–14. doi:10.1093/hmg/3.12.2103. PMID 7881406.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - García-Planells J, Burguera JA, Solís P; et al. (2005). "Ancient origin of the CAG expansion causing Huntington disease in a Spanish population". Hum. Mutat. 25 (5): 453–9. doi:10.1002/humu.20167. PMID 15832309.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Almqvist EW, Elterman DS, MacLeod PM, Hayden MR (2001). "High incidence rate and absent family histories in one quarter of patients newly diagnosed with Huntington disease in British Columbia". Clin. Genet. 60 (3): 198–205. PMID 11595021.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) -
Avila-Giron R. (1973). "Medical and Social Aspects of Huntington's chorea in the state of Zulia, Venezuela". Advances in Neurology. 1: 261–6.
{{cite journal}}: Cite has empty unknown parameter:|month=(help) - "The brief history of HD" (PDF). The HOPES Timeline - A Brief History of Huntington's Disease. standford hopes. 2005-04-05. Retrieved 2008-08-10.
- Okun MS (2003). "Huntington's disease: what we learned from the original essay". Neurologist. 9 (4): 175–9. doi:10.1097/01.nrl.0000080952.78533.1f. PMID 12864927.
{{cite journal}}: Unknown parameter|month=ignored (help) - Lund, Johan Christian (1860), "Chorea Sti Viti i Sætersdalen. Uddrag af Distriktslæge J. C. Lunds Medicinalberetning for 1860", Beretning om Sundhedstilstanden, Norway: 137–138
- Vessie, P.R. (1932), "On the transmission of Huntington's chorea for 300 years – the Bures family group", Nervous and Mental Disease, 76 (6), Baltimore: 553–573
- La Spada AR, Roling DB, Harding AE; et al. (1992). "Meiotic stability and genotype-phenotype correlation of the trinucleotide repeat in X-linked spinal and bulbar muscular atrophy". Nat. Genet. 2 (4): 301–4. doi:10.1038/ng1292-301. PMID 1303283.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - "Achievements of Hereditary Disease Foundation". Achievements of Hereditary Disease Foundation. Hereditary Disease Foundation. Retrieved 2008-08-10.
- "HDA research news—medical research into treatment & prevention on hda.org.uk". Retrieved 2008-08-10.
- "Alice's Restaurant". IMDB. Retrieved 2008-08-30.
- "ER (1994)". IMDB. Retrieved 2008-08-30.
- "Private Practice (2007)". IMDB. Retrieved 2008-08-30.
- "Everwood (2002)". IMDB. Retrieved 2008-08-30.
- "All Saints (1998)". IMDB. Retrieved 2008-08-30.
- "House M.D." IMDB. Retrieved 2008-08-30.
- "US Senate s. resolution 531" (PDF). S. Res. 531. US Senate. 2008-04-06. Retrieved 2008-08-10.
- Chapman MA (1990). "Predictive testing for adult-onset genetic disease: ethical and legal implications of the use of linkage analysis for Huntington disease". Am. J. Hum. Genet. 47 (1): 1–3. PMC 1683745. PMID 2140926.
{{cite journal}}: Unknown parameter|month=ignored (help) - Huggins M, Bloch M, Kanani S; et al. (1990). "Ethical and legal dilemmas arising during predictive testing for adult-onset disease: the experience of Huntington disease". Am. J. Hum. Genet. 47 (1): 4–12. PMC 1683755. PMID 1971997.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - "Guidelines for the molecular genetics predictive test in Huntington's disease. International Huntington Association (IHA) and the World Federation of Neurology (WFN) Research Group on Huntington's Chorea". Neurology. 44 (8): 1533–6. 1994. PMID 8058167.
{{cite journal}}: Unknown parameter|month=ignored (help) - Tibben A, Vegter-van der Vlis M, Skraastad MI; et al. (1992). "DNA-testing for Huntington's disease in The Netherlands: a retrospective study on psychosocial effects". Am. J. Med. Genet. 44 (1): 94–9. doi:10.1002/ajmg.1320440122. PMID 1387764.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Bloch M, Hayden MR (1990). "Opinion: predictive testing for Huntington disease in childhood: challenges and implications". Am. J. Hum. Genet. 46 (1): 1–4. PMC 1683548. PMID 2136787.
{{cite journal}}: Unknown parameter|month=ignored (help) - Binedell J, Soldan JR, Scourfield J, Harper PS (1996). "Huntington's disease predictive testing: the case for an assessment approach to requests from adolescents". J. Med. Genet. 33 (11): 912–8. PMC 1050784. PMID 8950670.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Wertz DC, Fanos JH, Reilly PR (1994). "Genetic testing for children and adolescents. Who decides?". JAMA. 272 (11): 875–81. PMID 8078166.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Hoffmann DE, Wulfsberg EA (1995). "Testing children for genetic predispositions: is it in their best interest?". J Law Med Ethics. 23 (4): 331–44. PMID 8715053.
- Gómez-Esteban JC, Lezcano E, Zarranz JJ; et al. (2007). "Monozygotic twins suffering from Huntington's disease show different cognitive and behavioural symptoms". Eur. Neurol. 57 (1): 26–30. doi:10.1159/000097006. PMID 17108691.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Kuliev A, Verlinsky Y (2005). "Place of preimplantation diagnosis in genetic practice". Am. J. Med. Genet. A. 134A (1): 105–10. doi:10.1002/ajmg.a.30635. PMID 15732064.
{{cite journal}}: Unknown parameter|month=ignored (help) - Davenport CB (1915). "Huntington's chorea in relation to heredity and eugenics". Proc. Natl. Acad. Sci. U.S.A. 1 (5): 283–5. PMC 1090803. PMID 16575999.
{{cite journal}}: Unknown parameter|month=ignored (help) - "BBC article: Genetic data banned for insurers". BBC. 2008-13-06. Retrieved 2008-08-10.
{{cite web}}: Check date values in:|date=(help) - Huntington's Disease Society of America "HDSA". http://www.hdsa.org/ Huntington's Disease Society of America website. HDSA. Retrieved 2008-08-10.
{{cite web}}: Check|url=value (help); External link in|work= - World Congress on Huntington's Disease "World Congress on Huntington's Disease website". Retrieved 2008-08-10.
{{cite web}}: Check|url=value (help) - Euro-HD Network "Euro-HD website". Retrieved 2008-08-10.
{{cite web}}: Check|url=value (help) - Huntington Project "Huntington Porject website". Retrieved 2008-08-10.
{{cite web}}: Check|url=value (help) - High-Q Foundation "HighQ foundation website". HighQ. Retrieved 2008-08-10.
{{cite web}}: Check|url=value (help) - Beal MF, Kowall NW, Ellison DW, Mazurek MF, Swartz KJ, Martin JB (1986). "Replication of the neurochemical characteristics of Huntington's disease by quinolinic acid". Nature. 321 (321): 168–71. doi:10.1038/321168a0. PMID 2422561.
{{cite journal}}: Cite has empty unknown parameter:|month=(help)CS1 maint: multiple names: authors list (link) - Brouillet E, Hantraye P, Ferrante RJ, Dolan R, Leroy-Willig A, Kowall NW, Beal MF (1995). "Chronic mitochondrial energy impairment produces selective striatal degeneration and abnormal choreiform movements in primates". Proc Natl Acad Sci USA. 92: 7105–7109. doi:10.1073/pnas.92.15.7105. PMID 7624378.
{{cite journal}}: Cite has empty unknown parameter:|month=(help)CS1 maint: multiple names: authors list (link) - Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, Bates GP (1996). "Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice". Cell. 87 (3): 493–506. doi:10.1016/S0092-8674(00)81369-0. PMID 8898202.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Carter RJ, Lione LA, Humby T, Mangiarini L, Mahal A, Bates GP, Dunnett SB, and Morton AJ (1999). "Characterization of Progressive Motor Deficits in Mice Transgenic for the Human Huntington's Disease Mutation". The Journal of Neuroscience. 19 (8): 3248–3257. PMID 10191337.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Marsh JL, Pallos J and Thompson LM (2003). "Fly models of Huntington's disease". Human Molecular Genetics. 12 (2): 187–193. doi:10.1093/hmg/ddg271. PMID 12925571.
{{cite journal}}: Cite has empty unknown parameter:|month=(help) -
"Science Daily article on HD monkey model". First Transgenic Monkey Model Of Huntington's Disease Developed. ScienceDaily. 2008-19-05. Retrieved 2008-08-10.
{{cite web}}: Check date values in:|date=(help) - Voisine C, Varma H, Walker N, Bates EA, Stockwell BR, Hart AC (2007). "Identification of potential therapeutic drugs for huntington's disease using Caenorhabditis elegans". PLoS ONE. 2 (6): e504. doi:10.1371/journal.pone.0000504. PMC 1876812. PMID 17551584.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - Lecerf JM, Shirley TL, Zhu Q; et al. (2001). "Human single-chain Fv intrabodies counteract in situ huntingtin aggregation in cellular models of Huntington's disease". Proc. Natl. Acad. Sci. U.S.A. 98 (8): 4764–9. doi:10.1073/pnas.071058398. PMID 11296304.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Miller TW, Zhou C, Gines S; et al. (2005). "A human single-chain Fv intrabody preferentially targets amino-terminal Huntingtin's fragments in striatal models of Huntington's disease". Neurobiol. Dis. 19 (1–2): 47–56. doi:10.1016/j.nbd.2004.11.003. PMID 15837560.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Wolfgang WJ, Miller TW, Webster JM; et al. (2005). "Suppression of Huntington's disease pathology in Drosophila by human single-chain Fv antibodies". Proc. Natl. Acad. Sci. U.S.A. 102 (32): 11563–8. doi:10.1073/pnas.0505321102. PMID 16061794.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Miller TW, Messer A (2005). "Intrabody applications in neurological disorders: Progress and future prospects". Mol. Ther. 12 (3): 394–401. doi:10.1016/j.ymthe.2005.04.003. PMID 15964243.
- Harper SQ, Staber PD, He X; et al. (2005). "RNA interference improves motor and neuropathological abnormalities in a Huntington's disease mouse model". Proc. Natl. Acad. Sci. U.S.A. 102 (16): 5820–5. doi:10.1073/pnas.0501507102. PMID 15811941.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Jamal Nasira; et al. (1995). "Targeted disruption of the Huntington's disease gene results in embryonic lethality and behavioral and morphological changes in heterozygotes". Cell. 81 (5): 811–23. doi:10.1016/0092-8674(95)90542-1.
{{cite journal}}: Explicit use of et al. in:|author=(help) - Jodi L. McBride; et al. (2008). "Artificial miRNAs mitigate shRNA-mediated toxicity in the brain: Implications for the therapeutic development of RNAi". PNAS. 105 (15): 5868–5873. doi:10.1073/pnas.0801775105. PMID 18398004.
{{cite journal}}: Explicit use of et al. in:|author=(help) - Miguel Díaz-Hernández, Jesús Torres-Peraza, Alejandro Salvatori-Abarca; et al. (October 19, 2005). "Full Motor Recovery Despite Striatal Neuron Loss and Formation of Irreversible Amyloid-Like Inclusions in a Conditional Mouse Model of Huntington's Disease". The Journal of Neuroscience. 25 (42): 9773–9781. doi:10.1523/JNEUROSCI.3183-05.2005. PMID 16237181.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - "Pig cell implants in Huntington's trial". WorldHealth.net. Retrieved 2008-05-15.
- "Trials for Huntington's disease at clinicaltrials.gov". Retrieved 2008-08-10.
- Mostert JP, Koch MW, Heerings M, Heersema DJ, De Keyser J (2008). "Therapeutic potential of fluoxetine in neurological disorders". CNS Neurosci Ther. 14 (2): 153–64. doi:10.1111/j.1527-3458.2008.00040.x. PMID 18482027.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
Bibliography
- Conomy, John P., M.D., J.D. "Dr. George Huntington and the Disease Bearing His Name". Retrieved 2008-08-15.
{{cite web}}: CS1 maint: multiple names: authors list (link) - Online Mendelian Inheritance in Man (OMIM): Huntington Disease - 143100
- Online Mendelian Inheritance in Man (OMIM): Huntingtons Disease-Like 2 - 606438
- Stevenson, Charles S. (1934). "A Biography of George Huntington, M.D" (Microsoft Word). Bulletin of the Institute of the History of Medicine. II (2). Johns Hopkins University. Retrieved 2007-12-05.
{{cite journal}}: Unknown parameter|month=ignored (help) - Vessie, P. R. (1932). "On the transmission of Huntington's chorea for 300 years—the Bures family group". Journal of Nervous and Mental Disease, Baltimore. 76: 553–573.
- Gillian Bates, Peter Harper, and Lesley Jones (2002). Huntington's Disease - Third Edition. Oxford: Oxford University Press. ISBN 0-19-851060-8.
{{cite book}}: CS1 maint: multiple names: authors list (link)
External links
Professional and research
- Huntington Project - worldwide umbrella organization for the clinical research efforts for HD.
- Huntington Study Group
- Huntington's Disease Research - Research abstracts on HD.
- Huntington's Outreach Project for Education, at Stanford (HOPES) - A layperson's guide to HD
- Worldwide Education and Awareness for Movement Disorders - HD section
- University College London Huntington's Disease Research
- TRACK-HD, an international observational study of HD
- University California and San Francisco Memory and Aging Center - HD Info
| Mental disorders (Classification) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| Diseases of the nervous system, primarily CNS | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inflammation |
| ||||||||||||||||||||||||
| Brain/ encephalopathy |
| ||||||||||||||||||||||||
| Both/either |
| ||||||||||||||||||||||||
| Non-Mendelian inheritance: anticipation | |||||
|---|---|---|---|---|---|
| Trinucleotide |
| ||||
| Tetranucleotide |
| ||||
| Pentanucleotide |
| ||||