This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 22:29, 21 July 2010 (Updating {{chembox}} (changes to watched fields) per Chem/Drugbox validation (report errors or bugs)). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 22:29, 21 July 2010 by CheMoBot (talk | contribs) (Updating {{chembox}} (changes to watched fields) per Chem/Drugbox validation (report errors or bugs))(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)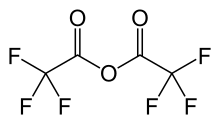
| |
| Names | |
|---|---|
| IUPAC name trifluoroacetic anhydride | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ECHA InfoCard | 100.006.349 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
SMILES
| |
| Properties | |
| Chemical formula | C4F6O3 |
| Molar mass | 210.031 g·mol |
| Density | 1.487 g/mL |
| Melting point | −65 °C (−85 °F; 208 K) |
| Boiling point | 40 °C (104 °F; 313 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Trifluoroacetic anhydride (TFAA) is the acid anhydride of trifluoroacetic acid. In particular, trifluoroacetic anhydride is the perfluorinated derivative of acetic anhydride. Like many acid anhydrides, it may be used to introduce the corresponding trifluoroacetyl group. The corresponding trifluoroacetyl chloride, is a gas, making it inconvenient to work with. Trifluoroacetic anhydride is the recommended desiccant for trifluoroacetic acid.
Preparation
Trifluoroacetic anhydride may be prepared from trifluoroacetic acid by dehydrating with excess α-halogenated acid chlorides. For example, with dichloroacetyl chloride:
- 2 CF3COOH + Cl2CHCOCl → (CF3CO)2O + Cl2CHCOOH + HCl
References
- Chai, Christina Li Lin; Armarego, W. L. F. (2003). Purification of laboratory chemicals (Google Books excerpt). Oxford: Butterworth-Heinemann. p. 376. ISBN 0-7506-7571-3.
{{cite book}}: CS1 maint: multiple names: authors list (link) - US 4595541