This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 15:03, 30 November 2010 (Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:Wi). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 15:03, 30 November 2010 by CheMoBot (talk | contribs) (Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:Wi)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) "CuTC" redirects here. For other uses, see CUTC (disambiguation).
| |
| Names | |
|---|---|
| IUPAC name Copper(I) thiophene-2-carboxylate | |
| Other names CuTC | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.161.358 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C5H3CuO2S |
| Molar mass | 190.68 g·mol |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Irritant (Xi) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
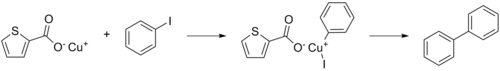
Copper(I)-thiophene-2-carboxylate or CuTC is a thiophene and a reagent in organic chemistry that especially promotes the Ullmann reaction between aryl halides.
References
- Copper(I) thiophene-2-carboxylate at Sigma-Aldrich
- Jwanro Hassan, Marc Sévignon, Christel Gozzi, Emmanuelle Schulz, and Marc Lemaire (2002). "Aryl-Aryl Bond Formation One Century after the Discovery of the Ullmann Reaction" (PDF). Chem. Rev. 102 (5): 1359–1470. doi:10.1021/cr000664r. PMID 11996540.
{{cite journal}}: CS1 maint: multiple names: authors list (link)