This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 13:28, 3 December 2010 (Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:Wi). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 13:28, 3 December 2010 by CheMoBot (talk | contribs) (Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:Wi)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)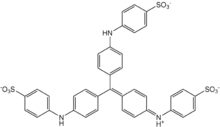
| |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.044.852 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C37H27N3Na2O9S3 |
| Molar mass | 799.814 g/mol |
| Solubility in water | ~ 300 g/l (20 °C) |
| Hazards | |
| NFPA 704 (fire diamond) |
 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Methyl blue, also known as Cotton blue, Helvetia blue, Acid blue 93, or C.I. 42780, is a chemical compound with the molecular formula C37H27N3Na2O9S3. It is used as a stain in histology. Methyl blue stains collagen blue in tissue sections. It is soluble in water and slightly soluble in ethanol. It can be used in the Mallory's connective tissue stain and Gömöri trichrome stain. It is used in differential staining. It can also be used to mediate electron transfer in microbial fuel cells.
Methyl blue is also used to stain fungal cell walls. Methyl blue is also available in mixture with water blue, under name Aniline Blue WS, Aniline blue, China blue, or Soluble blue.
Methyl blue should not be confused with methyl violet or methylene blue, two other stains.
Methyl blue is also used to treat thallium poisoning. It binds to the thallium.
See also
- Potassium ferrocyanide
- Potassium ferricyanide
- Methylene blue
- Egyptian Blue
- Han Purple
- Gentian violet
- Fluorescein
References
| This article does not cite any sources. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Methyl blue" – news · newspapers · books · scholar · JSTOR (October 2007) (Learn how and when to remove this message) |
| Microbial and histological stains | |
|---|---|
| Iron/hemosiderin | |
| Lipids | |
| Carbohydrates | |
| Amyloid | |
| Bacteria | |
| Connective tissue | |
| Other | |
| Tissue stainability | |