This is an old revision of this page, as edited by Beetstra (talk | contribs) at 11:04, 12 December 2010 (Script assisted update of identifiers from ChemSpider, CommonChemistry and FDA for the Chem/Drugbox validation project - Updated: {{cascite}} {{fdacite}} StdInChI StdInChIKey.). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 11:04, 12 December 2010 by Beetstra (talk | contribs) (Script assisted update of identifiers from ChemSpider, CommonChemistry and FDA for the Chem/Drugbox validation project - Updated: {{cascite}} {{fdacite}} StdInChI StdInChIKey.)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Bromodeoxyuridine" – news · newspapers · books · scholar · JSTOR (June 2009) (Learn how and when to remove this message) |
 | |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.000.378 |
| MeSH | Bromodeoxyuridine |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
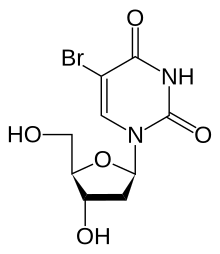
| Chemical formula | C9H11BrN2O5 |
| Molar mass | 307.098 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Bromodeoxyuridine (5-bromo-2-deoxyuridine, BrdU) is a synthetic nucleoside that is an analogue of thymidine. BrdU is commonly used in the detection of proliferating cells in living tissues.
BrdU can be incorporated into the newly synthesized DNA of replicating cells (during the S phase of the cell cycle), substituting for thymidine during DNA replication. Antibodies specific for BrdU can then be used to detect the incorporated chemical (see immunohistochemistry), thus indicating cells that were actively replicating their DNA. Binding of the antibody requires denaturation of the DNA, usually by exposing the cells to acid or heat.
Because BrdU can replace thymidine during DNA replication, it can cause mutations, and its use is therefore potentially a health hazard.
See also
External links
- BrdU incorporation during DNA replication - Jiayang Chien, Wellesley College
- BrdU at OpenWetWare, the bioscience wiki
This biochemistry article is a stub. You can help Misplaced Pages by expanding it. |