This is an old revision of this page, as edited by Citation bot (talk | contribs) at 12:07, 27 December 2010 (Citations: added: last1, first1, last2, first2, last3, first3, last4, first4. Tweaked: title, year, journal, volume, pages. stone). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 12:07, 27 December 2010 by Citation bot (talk | contribs) (Citations: added: last1, first1, last2, first2, last3, first3, last4, first4. Tweaked: title, year, journal, volume, pages. stone)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)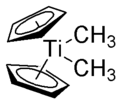 | |
 | |
| Names | |
|---|---|
| IUPAC name bis(η-cyclopentadienyl)dimethyltitanium | |
| Other names dimethyl titanocene | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ECHA InfoCard | 100.204.841 |
SMILES
| |
| Properties | |
| Chemical formula | C12H16Ti |
| Molar mass | 208.13 g/mol |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Irritant, incompatible with water and oxidizing agents |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
The Petasis reagent (not to be confused with the Petasis reaction) is dimethyl titanocene, Cp2TiMe2, readily prepared by the reaction of methylmagnesium chloride or methyllithium with titanocene dichloride:
- Cp2TiCl2 + 2 "Me" → Cp2TiMe2 + 2 Cl
It is used for transforming carbonyl groups to terminal alkenes, much like the Tebbe reagent or Wittig reaction. Unlike the Wittig reaction, the Petasis reagent can react with a wide range of carbonyls, including aldehydes, ketones and esters. The Petasis reagent is also more air stable than the Tebbe reagent, and can be isolated as a pure solid, or used directly as a solution in toluene-THF.
The active olefinating reagent, Cp2TiCH2, is prepared by heating the Petasis reagent in toluene or THF to 60 °C.
The reaction mechanism is very similar to that of the Tebbe reagent, first yielding a titanium carbene which forms a four membered oxatitanacyclo and than releases the terminal alkene.
References
- N. A. Petasis and E. I. Bzowej (1990). "Titanium-mediated carbonyl olefinations. 1. Methylenations of carbonyl compounds with dimethyltitanocene". J. Am. Chem. Soc. 112 (17): 6392–6394. doi:10.1021/ja00173a035.
- Payack, J. F.; Hughes, D. L.; Cai, D.; Cottrell, I. F.; Verhoeven, T. R. (2002). "Dimethyltitanocene". Organic Syntheses. 79: 19
{{cite journal}}: CS1 maint: multiple names: authors list (link). - Claus, K.; Bestian, H. (1962). "Über die Einwirkung von Wasserstoff auf einige metallorganische Verbindungen und Komplexe". Justus Liebigs Ann. Chem. 654: 8. doi:10.1002/jlac.19626540103.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Hartley, R. C.; Li, J.; Main, C. A.; McKiernan, G. J. (2007). "Titanium carbenoid reagents for converting carbonyl groups into alkenes". Tetrahedron. 63: 4825–4864. doi:10.1016/j.tet.2007.03.015.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Meurer, Eduardo Cesar; Santos, Leonardo Silva; Pilli, Ronaldo Aloise; Eberlin, Marcos N. (2003). "Probing the Mechanism of the Petasis Olefination Reaction by Atmospheric Pressure Chemical Ionization Mass and Tandem Mass Spectrometry". Organic Letters. 5: 1391. doi:10.1021/ol027439b.