This is an old revision of this page, as edited by Beetstra (talk | contribs) at 08:32, 9 March 2011 (Script assisted update of identifiers from ChemSpider, CommonChemistry and FDA for the Chem/Drugbox validation project - Updated: ChEMBL.). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 08:32, 9 March 2011 by Beetstra (talk | contribs) (Script assisted update of identifiers from ChemSpider, CommonChemistry and FDA for the Chem/Drugbox validation project - Updated: ChEMBL.)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| This article does not cite any sources. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Phosphoserine" – news · newspapers · books · scholar · JSTOR (December 2009) (Learn how and when to remove this message) |
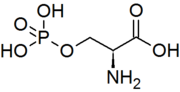
| |

| |
| Names | |
|---|---|
| IUPAC name (S)-2-Amino-3-(phosphonooxy)propionic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.352 |
| MeSH | Phosphoserine |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C3H8NO6P |
| Molar mass | 185.07 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Phosphoserine is an ester of serine and phosphoric acid. Phosphoserine is a component of many proteins as the result of posttranslational modifications. The phosphorylation of the alcohol functional group in serine to produce phosphoserine is catalyzed by various types of kinases.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |