This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 13:56, 14 April 2011 (Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report errors). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 13:56, 14 April 2011 by CheMoBot (talk | contribs) (Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report errors)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)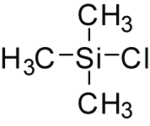
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name Trimethylsilyl chloride | |||
| Other names
Chlorotrimethylsilane TMSCl Trimethylchlorosilane TMCS | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.819 | ||
| PubChem CID | |||
| RTECS number |
| ||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C3H9SiCl | ||
| Molar mass | 108.64 g/mol | ||
| Appearance | Colorless liquid, fumes in moist air | ||
| Density | 0.856 g/cm, liquid | ||
| Melting point | −40 °C (233.2 K) | ||
| Boiling point | 57 °C (330.2 K) | ||
| Solubility in water | reacts | ||
| Structure | |||
| Molecular shape | tetrahedral at Si | ||
| Hazards | |||
| NFPA 704 (fire diamond) |
 | ||
| Flash point | −28 °C | ||
| Related compounds | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Trimethylsilyl chloride, also known as chlorotrimethylsilane is a silyl halide, with a variety of different uses in chemistry. It has the formula (CH3)3SiCl, and under standard conditions it is a colourless liquid, which is stable in the absence of water. It can be prepared from silicon tetrachloride by nucleophillic substitution of three of the chloride groups with a nucleophillic methyl source such as methyllithium, however the compound is readily available commercially.
Uses
Trimethylsilyl chloride has variety of uses in chemistry, both as a source of the trimethylsilyl group, and as an anhydrous source of chlorides. Functional groups such as alcohols and amines readily undergo reaction with trimethylsilyl chloride, giving trimethylsilyl ethers and trimethylsilyl amines. These new groups can be used as protecting groups for the original functional group, however the lability of the trimethylsilyl group restricts their utility. Trimethylsilylation can also be used to increase the volatility of a compound, enabling gas chromatography of normally non-volatile substances such as glucose. Trimethylsilyl chloride also reacts with metal acetylides to give trimethylsilyl alkynes, which are useful protected forms of alkynes.
The reaction of trimethylsilyl chloride with alcohols gives rise to an equivalent of hydrogen chloride. This reaction can be exploited to create anhydrous solutions of hydrochloric acid in alcohols, which find use in the mild synthesis of esters and acetals from carboxylic acids and ketones respectively.
In the presence of triethylamine and lithium diisopropylamide, enolisable aldehydes, ketones and esters are converted to trimethylsilyl enol ethers. Despite their hydrolytic instability, these compounds have found wide application in organic chemistry; oxidation of the double bond by epoxidation or dihydroxylation can be used to return the original carbonyl group with an alcohol group at the alpha carbon. The trimethylsilyl enol ethers can also be used as masked enolate equivalents in the Mukaiyama aldol addition.
Trimethylsilyl chloride can also be used as a starting material to prepare other trimethylsilyl halides and pseudohalides, such as:
- Trimethylsilyl fluoride
- Trimethylsilyl bromide
- Trimethylsilyl iodide
- Trimethylsilyl cyanide
- Trimethylsilyl azide
- Trimethylsilyl trifluoromethanesulfonate (TMSOTf)
These compounds are produced by an exchange reaction between trimethylsilyl chloride and a salt of the (pseudo)halide (MX):
- MX + Me3Si-Cl → MCl + Me3Si-X
Trimethylsilyl chloride is also used to silanize laboratory glassware, making the surfaces more lipophilic.
References
- L. Birkofer and P. Wegner (1988). "Trimethylsilyl azide". Organic Syntheses; Collected Volumes, vol. 6, p. 1030.
- Such as in Norbert Zander and Ronald Frank (2005). "The use of polystyrylsulfonyl chloride resin as a solid supported condensation reagent for the formation of esters: Synthesis of N--L-aspartic acid; α tert-butyl ester, β-(2-ethylphenyl]amino]ethyl ester". Organic Syntheses. 81: 235.

