This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 09:39, 18 April 2011 (Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 09:39, 18 April 2011 by CheMoBot (talk | contribs) (Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation ()(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) | |
| Names | |
|---|---|
| IUPAC name 2-hydroxybutane-1,2,4-tricarboxylic acid | |
| Other names
Homocitric acid Homocitrate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
SMILES
| |
| Properties | |
| Chemical formula | C7H10O7 |
| Molar mass | 206.15 g/mol |
| Appearance | colorless solid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Homocitric acid is an organic compound with the formula HOC(CO2H)(CH2CO2H)(C2H4CO2H). This tricarboxylic acid occurs naturally as a component of the iron-molybdenum cofactor of certain nitrogenase proteins. Biochemists often refer to this cofactor as homocitrate, which is the conjugate bases that predominate in neutral aqueous solutions of this species.
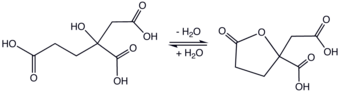
The molecule is related to citric acid by the addition of one methylene group, which is implied with the term "homo." Unlike citric acid, homocitric acid is chiral. The acid exists in equilibrium with the lactone.
References
- Douglas C. Rees "Great Metalloclusters in Enzymology" Annual Reviews of Biochemistry 2002, volume 71, pp. 221–46. doi:10.1146/annurev.biochem.71.110601.135406
This biochemistry article is a stub. You can help Misplaced Pages by expanding it. |