This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 12:14, 19 April 2011 (Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 12:14, 19 April 2011 by CheMoBot (talk | contribs) (Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation ()(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) The correct title of this article is Benzocinnoline. The substitution or omission of any brackets is due to technical restrictions.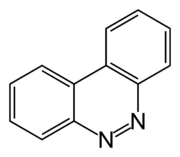
| |
| Names | |
|---|---|
| IUPAC name Benzocinnoline | |
| Other names Diphenylenazone; phenazone; 9,10-diazaphenanthrene; 2,2'-azobiphenyl; 3,4-benzocinnoline; 5,6-phenanthroline | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
SMILES
| |
| Properties | |
| Chemical formula | C12H8N2 |
| Molar mass | 180.21 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Benzocinnoline is a tricyclic organic compound with the formula C12H8N2. Formally this species is derived by oxidative dehydrogenation of 2,2'-diaminobiphenyl. This heterocycle reacts with iron carbonyls to form C12H8N2Fe2(CO)6.
See also
References
- R. P. Bennett, "Iron Carbonyl Complexes of Azo Compounds" Inorganic Chemistry, Volume 9, pp. 2184-6 (1970) (description of the first iron carbonyl derivative)
This article about a heterocyclic compound is a stub. You can help Misplaced Pages by expanding it. |