This is an old revision of this page, as edited by Christian75 (talk | contribs) at 10:27, 22 April 2011 (Clean up, typos fixed: eg. → e.g. using AWB). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 10:27, 22 April 2011 by Christian75 (talk | contribs) (Clean up, typos fixed: eg. → e.g. using AWB)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)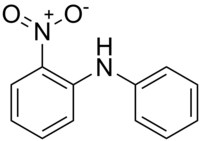 | |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.003.953 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C12H10N2O2 |
| Molar mass | 214.224 g·mol |
| Melting point | 74 - 75 °C |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
2-Nitrodiphenylamine, also called NDPA, 2-NDPA, 2NO2DPA, Sudan Yellow 1339, C.I. 10335, CI 10335, phenyl 2-nitrophenylamine, 2-nitro-N-phenylaniline, or N-phenyl-o-nitroaniline, is an organic chemical, a nitrated aromatic amine, a derivate of diphenylamine. Its chemical formula is C12H10N2O2, or C6H5NHC6H4NO2. It is a red crystalline solid, usually in form of flakes or powder, with melting point of 74-76 °C and boiling point of 346 °C. It is polar but hydrophobic.
2-Nitrodiphenylamine is used as a stabilizer for synthetic rubbers, explosives, propellants (e.g. in Otto fuel II, smokeless powders, in some US Army double-base propellants in solid rockets, and in other applications involving nitric acid esters), plastics, and lubricants. It is also an intermediate for organic synthesis, and a civilian solvent dye.
In some explosives, it is used to control the explosion. One of its major uses is to control the explosion rate of propylene glycol dinitrate.
As a stabilizer, its major role is to eliminate the acidic nitrates and nitrogen oxides produced by gradual decomposition of nitric acid esters, which would otherwise autocatalyze further decomposition. Its amount is usually 1-2% of the mixture; higher amounts than 2% degrade the propellant's ballistic properties. The amount of the stabilizer depletes with time; remaining content of less than 0.5% (with initial 2% content) requires increased surveillance of the munition, with less than 0.2% warranting immediate disposal, as the depletion of the stabilizer may lead to autoignition of the propellant.
Other stabilizers of a similar nature are 4-nitrodiphenylamine, N-nitrosodiphenylamine, N-methyl-p-nitroaniline, and diphenylamine.