This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 16:30, 12 May 2011 (Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 16:30, 12 May 2011 by CheMoBot (talk | contribs) (Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation ()(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)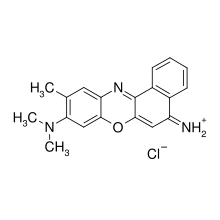 | |
| Names | |
|---|---|
| IUPAC name (9-dimethylamino-10-methyl-benzophenoxazin-5-ylidene)ammonium chloride | |
| Other names 9-(Dimethylamino)-5-imino-10-methyl-5H-benzo(a)phenoxazine hydrochloride; Cresole Violet | |
| Identifiers | |
| 3D model (JSmol) | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C19H18ClN3O |
| Molar mass | 339.8187 |
| Hazards | |
| Flash point | 245.5 °C |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Cresyl violet is an organic compound with the chemical formula C19H18ClN3O. It is used in biology and medicine as a histological stain. Cresyl violet is an effective and reliable stain used for light microscopy sections. Initially, tissue sections are ‘defatted’ by passing through graded dilutions of ethanol. Then, rehydrated by passing back through decreasing concentrations of ethanol. Lastly, back into water. The ethanol solutions act to differentiate the stain, causing myelin and other components to lose color whereas perikarya retain the color.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |